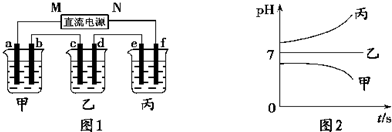
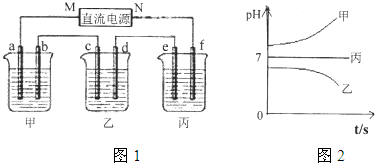
���𰸡�
�������������c�缫����������16g�������к���Cu
2+��������ӵĹ����֪��BΪCuSO
4������pH���䣬��CΪ�����ƻ�����أ�����pH������AΪKOH��NaOH��
��1������c�缫ͭ���ӵõ��ӣ���cΪ��������MΪ��Դ��������bΪ����������Һ��OH
-�ŵ磻
��2��e�缫�������ӷŵ���������������Cu��2e
-��H
2�������㣻
��3�����ձ���Ϊ���Ե缫�������ͭ��Һ��
��4��B��Һ�еĽ�������ȫ�������������Ϊ���ᣬ�ܵ�⣻
��5�����е����Ϊ�����ƻ�����أ�ʵ��Ϊ���ˮ��
����⣺�������c�缫����������16g�������к���Cu
2+��������ӵĹ����֪��BΪCuSO
4������pH���䣬��CΪ�����ƻ�����أ�����pH������AΪKOH��NaOH��
��1������c�缫ͭ���ӵõ��ӣ���cΪ��������MΪ��Դ��������bΪ����������Һ��OH
-�ŵ磬�缫��ӦΪ4OH
--4e
-=O
2��+2H
2O���ʴ�Ϊ������4OH
--4e
-=O
2��+2H
2O��
��2��e�缫�������ӷŵ�����������n��Cu��=

=0.25mol����Cu��2e
-��H
2����֪���ɱ�������������Ϊ0.25mol×22.4L/mol=5.6L���ʴ�Ϊ��5.6L��
��3�����ձ���Ϊ���Ե缫�������ͭ��Һ���ܷ�ӦΪ2CuSO
4+2H
2O

2Cu+O
2��+2H
2SO
4��
�ʴ�Ϊ��2CuSO
4+2H
2O

2Cu+O
2��+2H
2SO
4��
��4��B��Һ�еĽ�������ȫ�������������Ϊ���ᣬ���ʱ��Һ�е������ӡ����������ӷŵ磬�ܼ�����⣬
�ʴ�Ϊ���ܣ����������Һ��
��5�����е����Ϊ�����ƻ�����أ�ʵ��Ϊ���ˮ���ɵ����غ��֪H
2O��2e
-��Cu����Ҫ�ָ�ԭ״����0.25mol×18g/mol=4.5gˮ���ʴ�Ϊ������4.5gˮ��
���������⿼����ԭ������ȷ�����ĵ缫��Ӧ����ⷴӦ��ͼ��ķ����ǽ����Ĺؼ���ע���������Ƴ������ʼ���Դ���������ǽ���ͻ�ƿڣ���Ŀ�Ѷ��еȣ�

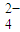 ��OH-
��OH-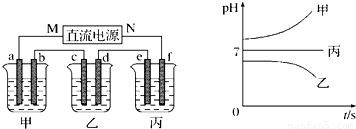
 =0.25mol����Cu��2e-��H2����֪���ɱ�������������Ϊ0.25mol×22.4L/mol=5.6L���ʴ�Ϊ��5.6L��
=0.25mol����Cu��2e-��H2����֪���ɱ�������������Ϊ0.25mol×22.4L/mol=5.6L���ʴ�Ϊ��5.6L�� 2Cu+O2��+2H2SO4��
2Cu+O2��+2H2SO4�� 2Cu+O2��+2H2SO4��
2Cu+O2��+2H2SO4��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�