
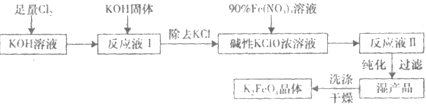
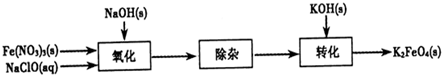
 ������أ�K2FeO4����һ�ּ�������������������ɱ���������ȥ�ǡ���ɫ������Ϊһ������͡���Ч����ɫ�����Ķ��ˮ����������ʮ���������ҹ��Ը������������ˮ�����е�Ӧ�õ��о�Ҳ�������룬��ȡ�ÿ�ϲ�ɹ����Ƚ�������Ʊ������Ǵ�������������������KOH��Һ��ͨ������Cl2�Ʊ�������ر�����Һ���ٷִμ���KOH���壬�õ��������ǿ���Ա�����Һ�������������Σ��ϳɸ�����أ�
������أ�K2FeO4����һ�ּ�������������������ɱ���������ȥ�ǡ���ɫ������Ϊһ������͡���Ч����ɫ�����Ķ��ˮ����������ʮ���������ҹ��Ը������������ˮ�����е�Ӧ�õ��о�Ҳ�������룬��ȡ�ÿ�ϲ�ɹ����Ƚ�������Ʊ������Ǵ�������������������KOH��Һ��ͨ������Cl2�Ʊ�������ر�����Һ���ٷִμ���KOH���壬�õ��������ǿ���Ա�����Һ�������������Σ��ϳɸ�����أ� 3Zn��OH��3+2Fe��OH��3+4KOH�õ�طŵ�ʱ�ĸ�����ӦʽΪ______�������·��5.418��1022������ͨ������������______g������ز��뷴Ӧ��
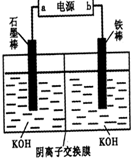
3Zn��OH��3+2Fe��OH��3+4KOH�õ�طŵ�ʱ�ĸ�����ӦʽΪ______�������·��5.418��1022������ͨ������������______g������ز��뷴Ӧ��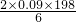 g=5.94g���ʴ�Ϊ��Zn+2OH--2e-=Zn��OH����5.94��
g=5.94g���ʴ�Ϊ��Zn+2OH--2e-=Zn��OH����5.94��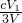 mol/L���ʴ�Ϊ��
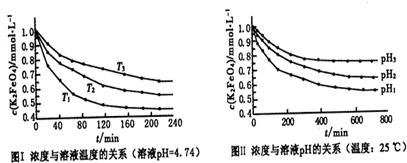
mol/L���ʴ�Ϊ��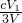 mol/L��
mol/L��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com