
| 1.6 |
| 32 |
| 64.22 |
| 0.05 |
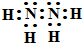 ��1molN2H4����4mol�ļ��Թ��ۼ�������Ϊ4NA�����ʴ�Ϊ��
��1molN2H4����4mol�ļ��Թ��ۼ�������Ϊ4NA�����ʴ�Ϊ��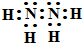 ��4NA��
��4NA��| 1.6 |
| 32 |
| 64.22 |
| 0.05 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��150 mL |
| B��200 mL |
| C��225 mL |
| D��250 mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����֪��N2��g��+2O2��g��=2NO2��g����H=+68kJ?mol-12C��s��+O2��g��=2CO��g����H=-221kJ?mol-1C��s��+O2��g��=CO2��g����H=-393.5kJ?mol-1��4CO��g��+2NO2��g��=4CO2��g��+N2��g����H=+1200kJ?mol-1 | ||||||||||||
| B����H+��aq��+OH-��aq��=H2O��l����H=-57.3 kJ?mol-1������0.1 mol HCl�������м���4.0 gNaOH���壬�ų���������5.73 kJ | ||||||||||||
C��ͨ�����ǰѲ�1 mol��̬������ij�ֹ��ۼ���Ҫ���յ��������ɸù��ۼ��ļ���
| ||||||||||||
| D��NH4HCO3��s���TNH3 ��g��+H2O��g��+CO2��g����H=+185.57 kJ?mol-1���Է����У�ԭ������ϵ���Է�������Ҷ����ӵķ���ת������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Mg | B��Al | C��Zn | D��Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
�� ͨ������·�߿ɺϳɣ�G����
ͨ������·�߿ɺϳɣ�G����
 ����д���Լ״���AΪԭ���Ʊ�
����д���Լ״���AΪԭ���Ʊ� �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���| HBr |
| NaOH��Һ |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH3COOH��NaOH��������� |
| B��CH3COOH��NaOH�����ʵ������ |
| C��NaOH���� |
| D��CH3COOH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����������ˮ���ȣ���ַ�Ӧ������������ˮ�����ʵ����ǣ�
����������ˮ���ȣ���ַ�Ӧ������������ˮ�����ʵ����ǣ�| A��3mol | B��4mol |
| C��5mol | D��2mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com