
�������������ͬ��������Һ����pH=3��CH3COOH��Һ ��pH=3������ ��pH=11�İ�ˮ��pH=11��NaOH��Һ������˵����ȷ����
A������������Һϡ��100������ ҺpH��С˳��>��>��>��
ҺpH��С˳��>��>��>��
B���ۺֱܷ͢��õ�Ũ�ȵ�������Һ�кͣ�����������Һ���������=��
C������ڷֱ�������þ�۷�Ӧ������H2��������<��
D���ں͢ۻ�ϣ� ���û����Һ��pH����7
���û����Һ��pH����7
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ���������ݸ�����һ12���¿���ѧ���������棩 ���ͣ��ƶ���
�ס��ҡ���Ϊ�������ʡ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ��
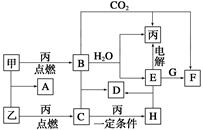
��ش��������⣺
(1)�û�ѧʽ��ʾ����Ϊ__________��HΪ__________��
(2)A�ĵ���ʽΪ________________________________��
(3)���E��ˮ��Һʱ��E��������________________________��
(4)д��B��C����D�Ļ�ѧ����ʽ��_____________________________��
д��E��G����F�����ӷ���ʽ��__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и�������һ12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������114����Ԫ�أ�λ�ڵ�7����IVA�壩�������й�X���ܾ��е����ʺ������ǣ� ��
A. X�Ƿǽ���Ԫ�� B. X��+2�ۻ������+4�ۻ������ȶ�
C. XO2 ����ǿ������ D. X���ȶ�����̬�⻯��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и߶�����ĩ��ѧ���������棩 ���ͣ������
�о��Ϳ���CO2��CO�Ĵ� �������ǻ�����������Դ����˫Ӯ�Ŀ��⡣
�������ǻ�����������Դ����˫Ӯ�Ŀ��⡣
��1��CO�����ںϳɼ״���������ɱ���ܱ������г���4molCO��8molH2���ڴ��������ºϳɼ״���CO��g��+2H2��g�� CH3OH��g��(��)��ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH��g��(��)��ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��

�ٸ÷�Ӧ���淴Ӧ����________��Ӧ��������ȡ����ȡ�����
����0.1Mpa ��100��������£��÷�Ӧ�ﵽƽ��ʱ�������Ϊ��ʼ���������_________���������������λС���㣩
�����¶Ⱥ��ݻ����������£�����ƽ����ϵ�г���4molCO���ﵽƽ��ʱCOת����________������������䡱��С������ƽ�ⳣ��K________������������䡱��С������
��2���ڷ�Ӧ(��)����Ҫ�õ�H2����Ӧ��Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ������֪��
��CH4��g��+ H2O��g��= CO��g��+3H2��g�� ��H=+206.2 kJ��mol-1
��CH4��g��+ CO2��g��= 2CO��g��+2H2��g�� ��H=+247.4 kJ��mol-1
��CH4��H2O��g����Ӧ����CO2��H2���Ȼ�ѧ����ʽΪ�� ��
��3���ڷ�Ӧ(��)���Ƶõ�CH3OH ��������ȼ�ϣ���������������ɼ���ȼ�ϵ�أ��������Һ��20����30����KOH��Һ�����ȼ�ϵ�طŵ�ʱ�������ĵ缫��ӦʽΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и߶�����ĩ��ѧ���������棩 ���ͣ�ѡ����
��֪25 ��ʱ��CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ������������100 mL��CaSO4������Һ�У�����200 mL 0.03 mol��L ��1 ��Na2SO4��Һ����Դ˹��̵�����������ȷ����(���Ի�Ϲ����е�����仯)
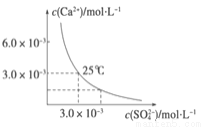
A����Һ������CaSO4������������Һ��c(SO42��)��ԭ����
B ��Һ������CaSO4��������Һ��c(Ca2�� )��c(SO 42��)����С
C����Һ��������������Һ��c(Ca2�� )��c(SO42��)����С
D����Һ��������������������Һ��c(SO42��)��ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и߶�����ĩ��ѧ���������棩 ���ͣ�ѡ����
����ʵ����������ݼ�¼����ȷ����( )
A. ��������ƽ����ʱ����NaOH������������ڵ�ֽ�ϣ��Ƶ�����Ϊ10.2 g
B. ��25 mL��ʽ�ζ�����ȡ���������Һ�����Ϊ16.60 mL
C. �ø���Ĺ㷺pH��ֽ��ϡ�����pH��3.2
D. ��10 mL��Ͳ��ȡNaCl��Һ�����Ϊ9.2 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и߶�����ĩ��ѧ���������棩 ���ͣ�ѡ����
���л�ѧ����ʽ�У�����ˮ�ⷴӦ���ǣ� ��
A��H2O��H2O H3O����OH��
H3O����OH��
B��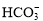 ��OH��
��OH�� H2O��
H2O��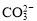
C��CO2��H2O H2CO3
H2CO3
D��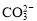 ��H2O
��H2O
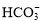 ��OH��
��OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ��һ12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͬ�����£��������ʵ�����ͬ�������Ȼ ( )
A�������Ϊ22��4L B ��
�� ������ͬ�����
������ͬ�����
C����˫ԭ�ӷ��� D��������ͬ��ԭ����Ŀ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�������ѧ��һ���ǰ�12���¿���ѧ���������棩 ���ͣ�ѡ����
ʵ���������Ƹ�ˮ��Ӧ��ʵ��ʱ,�õ���������ҩƷ��:���ԹܼС������ӡ���С��������ֽ�����в������ձ�������������ʯ�������Უ��Ƭ����ҩ��.
A���٢ڢۢ� B���ڢۢܢޢ�
B���ڢۢܢޢ�  C���ۢܢ��� D���ڢݢߢ��
C���ۢܢ��� D���ڢݢߢ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com