
(l2·Ö£©ĻĀ±ķŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£¬øł¾ŻĖłøųµÄ10 ÖÖŌŖĖŲ£¬»Ų“šĻĀĮŠ
ĪŹĢā”£
£Ø1£©·Ē½šŹōŠŌ×īĒæµÄŌŖĖŲŹĒ £»
£Ø2£©Ne Ō×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ £»
£Ø3£©C ÓėN ÖŠ£¬Ō×Ó°ė¾¶½ĻŠ”µÄŹĒ £»
£Ø4£©ĀČĖ®¾ßÓŠĘÆ°××÷ÓĆ£¬ŹĒÓÉÓŚĘäÖŠŗ¬ÓŠ £ØĢī”°HCl”±»ņ”°HClO”±)
£Ø5£©ŌŖĖŲ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļÖŠ£¬¼īŠŌ×īĒæµÄŹĒ £ØĢī»ÆѧŹ½£©£¬³ŹĮ½ŠŌµÄŹĒ £ØĢī»ÆѧŹ½£©£»
£Ø6£©ŌŖĖŲ¹čµÄŃõ»ÆĪļ³£ÓĆÓŚÖĘŌģ £ØĢīŅ»ÖÖøߊŌÄܵÄĻÖ“śĶØѶ²ÄĮĻµÄ
Ćū³Ę£©£»
£Ø7£©ÓŅĶ¼ ĪŖijӊ»śĪļµÄĒņ¹÷Ä£ŠĶ£ØĘäÖŠ
ĪŖijӊ»śĪļµÄĒņ¹÷Ä£ŠĶ£ØĘäÖŠ “ś±ķĒāŌ×Ó“ś±ķ
“ś±ķĒāŌ×Ó“ś±ķ Ģ¼Ō×Ó£©£¬øĆÓŠ»śĪļÖŠĢ¼ŌŖĖŲÓėĒāŌŖĖŲµÄÖŹĮæ±Čm(C):m(H)£½ ”££ØĻą¶ŌŌ×ÓÖŹĮæC-12”¢H-l)
Ģ¼Ō×Ó£©£¬øĆÓŠ»śĪļÖŠĢ¼ŌŖĖŲÓėĒāŌŖĖŲµÄÖŹĮæ±Čm(C):m(H)£½ ”££ØĻą¶ŌŌ×ÓÖŹĮæC-12”¢H-l)
£Ø8£©Ć¾ŹĒÖĘŌģĘū³µ”¢·É»ś”¢»š¼żµÄÖŲŅŖ²ÄĮĻ”£Š“³ö¹¤ŅµÉĻµē½āČŪČŚĀČ»ÆĆ¾»ńµĆ½šŹōĆ¾µÄ»Æѧ·½³ĢŹ½ ”£
(l)F»ņ·ś (2) 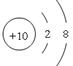
(3)N (4) HClO (5)NaOH Al(OH)3
(6)¹āµ¼ĻĖĪ¬ (7)6:l (8) MgCl2 Mg+Cl2”ü
Mg+Cl2ӟ
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©øł¾ŻŌŖĖŲÖÜĘŚ±ķæÉÖŖ£¬·Ē½šŹōŠŌ×īĒæµÄŌŖĖŲŹĒF”£
£Ø2£©NeµÄŌ×ÓŠņŹżŹĒ10£¬ĖłŅŌŌ×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ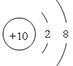 .
.
£Ø3£©Ķ¬ÖÜĘŚ×Ō×óĻņÓŅŌ×Ó°ė¾¶ŹĒÖš½„¼õŠ”µÄ£¬ĖłŅŌC ÓėN ÖŠ£¬Ō×Ó°ė¾¶½ĻŠ”µÄŹĒN”£
£Ø4£©ĀČĘųČÜÓŚĖ®Éś³ÉµÄ“ĪĀČĖį¾ßÓŠĘư׊Ō
£Ø5£©½šŹōŠŌŌ½Ē棬×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļµÄ¼īŠŌŌ½Ē棬ĖłŅŌ¼īŠŌ×īĒæµÄŹĒĒāŃõ»ÆÄĘ”£¶ųĒāŃõ»ÆĀĮŹĒĮ½ŠŌĒāŃõ»ÆĪļ”£
£Ø6£©ŌŖĖŲ¹čµÄŃõ»ÆĪļ¶žŃõ»Æ¹č³£ÓĆÓŚÖĘŌģ¹āµ¼ĻĖĪ¬”£
£Ø7£©øł¾ŻÄ£ŠĶæÉÖŖøĆÓŠ»śĪļŹĒŅŅĻ©£ØCH2=CH2£©£¬ĘäÖŠĢ¼ŌŖĖŲÓėĒāŌŖĖŲµÄÖŹĮæ±Čm(C):m(H)£½24©U4£½6:l”£
£Ø8£©Ć¾ŹĒ»īĘĆµÄ½šŹō£¬Ó¦øĆÓƵē½ā·ØŅ±Į¶£¬ĖłŅŌ¹¤ŅµÉĻµē½āČŪČŚĀČ»ÆĆ¾»ńµĆ½šŹōĆ¾µÄ»Æѧ·½³ĢŹ½ŹĒMgCl2 Mg+Cl2”ü”£
Mg+Cl2ӟӣ
æ¼µć£ŗæ¼²éŌŖĖŲÖÜĘŚ±ķµÄ½į¹¹ŅŌ¼°ŌŖĖŲÖÜĘŚĀɵÄÓ¦øĆÓ¦ÓĆ”£
µćĘĄ£ŗ±¾ĢāµÄøł¾ŻŹĒŹģĮ·ÕĘĪÕŌŖĖŲÖÜĘŚ±ķµÄ½į¹¹ŅŌ¼°ŌŖĖŲÖÜĘŚĀÉ£¬²»ÄÜĮé»īŌĖÓĆ”£


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com