
���жԻ�ѧƽ���ƶ��ķ����У�����ȷ���� �� ��
��1���Ѵ�ƽ��ķ�Ӧ

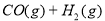 �������ӷ�Ӧ�����ʵ���ʱ��ƽ��һ��������Ӧ�����ƶ�
�������ӷ�Ӧ�����ʵ���ʱ��ƽ��һ��������Ӧ�����ƶ�
��2���Ѵ�ƽ��ķ�Ӧ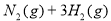

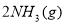 ��������
�������� ��Ũ��ʱ��ƽ��������Ӧ�����ƶ���
��Ũ��ʱ��ƽ��������Ӧ�����ƶ��� ��ת����һ������
��ת����һ������
��3��������μӵķ�Ӧ��ƽ��ʱ������С��Ӧ���ݻ�ʱ��ƽ��һ���������������ķ����ƶ�
��4��������μӵķ�Ӧ��ƽ����ں�ѹ��Ӧ���г���ϡ�����壬ƽ��һ�����ƶ�
A����1����4�� B����1����2����3��
C����2����3����4�� D����1����2����3����4��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016�����ʡ������ѧ�ڵ�����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��0.2mol/LCH3COOK��0.1mol/L����������Ϻ���Һ�������������ʵ���Ũ�ȵĹ�ϵ��ȷ����
A��c(CH3COO-)=c(Cl-)=c(H+)��c(CH3COOH)
B��c(CH3COO-)=c(Cl-)��c(CH3COOH)��c(H+��
C��c(CH3COO-)��c(Cl-)��c(H+)��c(CH3COOH��
D��c(CH3COO-)��c(Cl-)��c(CH3COOH)��c(H+��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫��ͷ��ɽ��ѧ��һ��12���¿���ѧ���������棩 ���ͣ�ѡ����
���з�Ӧ�У�HCl��Ϊ����������
A��HCl+AgNO3 �THNO3+AgCl�� B��Mg+2HCl �TMgCl2+H2��
C��MnO2+4HCl �TMnCl2+Cl2��+2H2O D��Au+HNO3+4HCl �THAuCl4+NO��+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶������У�������ѧ�Ծ��������棩 ���ͣ�ѡ����
��ӦA(g)+3B(g) 2C(g)+2D(g)�ڲ�ͬ����²�÷�Ӧ���ʣ����з�Ӧ������
2C(g)+2D(g)�ڲ�ͬ����²�÷�Ӧ���ʣ����з�Ӧ������
A��v��A��=0.5mol��L•min�� B��v��B��=0.9mol��L•min��
C��v��C��=1.2mol��L•min�� D��v��D��=0.4mol��L•min��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶��϶����¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
���к͵ζ��ķ����ⶨNaOH��Na2CO3�Ļ����Һ��NaOH�ĺ����������ڻ��Һ�м��������BaCl2��Һ��ʹNa2CO3��ȫת���BaCO3������Ȼ���ñ�����ζ�(��֪�������ָʾ����ɫ��pH��Χ���ټ���3.1��4.4 �ڼ���4.4��6.2 �۷�̪8.2��10)��
��1���ζ�ʱӦѡ�� ��ָʾ����
��2���жϵ���ζ��յ��ʵ�������� ��
��3�����в����ᵼ���ռ���Ʒ��NaOH�����ⶨֵƫ�ߵ���
A����ƿ������ˮϴ��δ�ô���Һ��ϴ B����ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ
C���ڵζ�ǰ�����ݣ��ζ���������ʧ D���ζ�ǰƽ�Ӷ������ζ��������Ӷ���
��4��Ϊ�ⶨij�ռ���Ʒ��NaOH�ĺ���������Ʒ������ΪNa2CO3����ijͬѧ��������ʵ�飺ȷ��ȡ5.0g��Ʒ���Ƴ�250mL��Һ��Ȼ������θ�ȡ���ƺõ��ռ���Һ20.00mL������������ˮϴ������ƿ�У��ֱ���������BaCl2��Һ��������ƿ�и�����1��2��ָʾ������Ũ��Ϊ0.2000mol��L-1�������Һ���еζ���������ݼ�¼���£�
ʵ���� | V(�ռ���Һ)/mL | V(HCl)/mL | |
������ | ĩ���� | ||
1 | 20.00 | 0.80 | 21.00 |
2 | 20.00 | 1.00 | 20.80 |
3 | 20.00 | 0.20 | 22.80 |
���ݱ������ݣ�������ռ���Ʒ�к�NaOH����������Ϊ %����С���������λ���֣�
��5����ij��Ʒ������NaOH��Na2CO3��NaHCO3�е�һ�ֻ�������ɣ�Ϊȷ������ɣ�ijͬѧ��������ʵ�飺
ȷ��ȡmg��Ʒ���Ƴ�250mL��Һ��ȡ���ƺõ���Һ20.00mL����ƿ�У�����2�η�̪��ָʾ������Ũ��Ϊcmol��L-1�������Һ���еζ����յ㣬���������Һv1ml��Ȼ���ٵμ�2�μ��ȼ�����Ũ��Ϊcmol��L-1�������Һ���еζ����յ㣬���������Һv2ml(v1��v2����Ϊ0)��
����v1��v2��ֵ��С�ж���Ʒ����ɣ��û�ѧʽ��ʾ����
��v1>v2 ��v1=v2 ��v1<v2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016���㽭ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����йص������Һ��˵����ȷ����
A��������ˮ�еμ�ŨH2SO4��KW����
B����NaAlO2��Һ�еμ�NaHCO3��Һ���г�������������
C���к͵���������ʵ���Ũ�ȵ�����ʹ��ᣬ�����ĵ��������Ƶ����ʵ�����ͬ
D��NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������и߶������в��Ի�ѧ�Ծ��������棩 ���ͣ������
�±��dz����£�Ũ��Ϊ0.01 mol��L-1��NaOH��Һ�뼸������λ�Ϻ�������
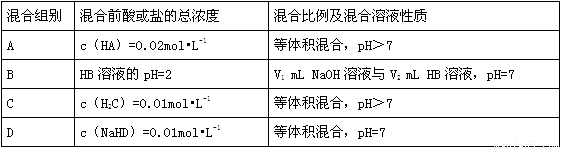
�ش��������⣺
��1��A����Һ�У�HA��A-��Na+��������Ũ���ɴ�С��˳��Ϊ________����pH=8����c��Na+��-c��A-��=______ mol•L-1����������֣���
��2����B����ҺpH=7����֪��V1____V2��
��3��C����Һ�У�pH��7��ԭ����________��
��4��0.01mol•L-1NaHD��Һ�У�ˮ�ĵ���ȣ��ѵ���������ʼ����֮�ȣ�=________��
��5����ƾ���������һ����ȷ��HA��HB��H2C��H2D�������������������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������и�һ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA���������ӵ������������й�˵������ȷ���ǣ� ��
A����״���£�2.24LCCl4���еķ�����Ϊ0.1NA
B��500mL1mol/L��K2SO4��Һ��K+�����ʵ���Ϊ2mol
C��25�棬101KPa�£�11.2LCO2��������ԭ�Ӹ���С��NA
D��2.7gAl������ϡ���ᷴӦ��ת��0.2NA������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������������������ѧ��һ��10�»�ѧ���������棩 ���ͣ�������
����m gij����X2,����Ħ������ΪM g��mol-1.�������ӵ�������NA��ʾ,��:
��1������������ʵ���Ϊ____________________mol.
��2������������ԭ������Ϊ_______________________��.
��3���������ڱ�״���µ����Ϊ____________________L.
��4������������1Lˮ��(�����Ƿ�Ӧ),����Һ�����ʵ���������Ϊ__________________.
��5������������ˮ���γ�VL��Һ,����Һ�����ʵ���Ũ��Ϊ__________________mol��L-1.
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com