
�ס�����ͬѧ�о�Na2SO3��Һ��FeCl3��Һ��Ӧ�������
| ���� | ���� | ���� |
| �� | ��2 mL 1 mol��L��1 FeCl3��Һ�м���һ������Na2SO3��Һ | ��Һ���ػ�ɫ��Ϊ���ɫ�����������̼�����ζ�������ݳ� |
(1)�����£�FeCl3��Һ��pH________7(�����������������)��
(2)�������ɫ������ԭ��
�ټ�ͬѧ��Ϊ����I����Һ�ʺ��ɫ����Ϊ������Fe(OH)3�����û�ѧƽ���ƶ�ԭ��������Һ�ʺ��ɫ��ԭ��___________________________��
����ͬѧ��Ϊ�����Ƿ�����������ԭ��Ӧ������Fe3����Fe2������д��Fe3����SO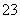 ��Ӧ�����ӷ���ʽ_____________________________________________
��Ӧ�����ӷ���ʽ_____________________________________________
________________________________________________________��
��ͬѧ�������ϵ�֪��
| 1.Fe2����SO 2.ī��ɫ��FeSO3���ɫ��FeCl3��Һ��Ϻ���Һ�ʺ��ɫ�� |
(3)��ͬѧΪ��ȷ����Һ�ʺ��ɫ��ԭ����������Fe(OH)3����Ʋ����������ʵ�飺
| ���� | ���� | ���� |
| �� | �ü�������䲽��I�еĺ��ɫ��Һ | ���֡������ЧӦ�� |
��ͬѧ��˵ó����ۣ���Һ�ʺ��ɫ����Ϊ������Fe(OH)3������ͬѧ��Ϊ��ͬѧ�ó����۵�֤����Ȼ���㣬��ͬѧ��������________��
(4)Ϊ��һ��ȷ��Na2SO3��Һ��FeCl3��Һ��Ӧ���������ͬѧ��Ʋ����������ʵ�飺
| ���� | ���� | ���� |
| �� | ��1 mol��L��1��FeCl3��Һ��ͨ��һ������SO2 | ��Һ�ɻ�ɫ��Ϊ���ɫ |
| �� | �ü�������䲽����еĺ��ɫ��Һ | û�г��֡������ЧӦ�� |
�پ����鲽����к��ɫ��Һ����Fe2��������Fe2��ѡ�õ��Լ���________(����ĸ)��
a��K3[Fe(CN)6]��Һ��b��KSCN��Һ��c��KMnO4��Һ
���������ӷ���ʽ�ͱ�Ҫ������˵��������г��ֺ��ɫ��ԭ��____________________��
(5)���ۣ�������ʵ���֪���ס�����ͬѧ���ֹ۵����ȷ��
������(1)FeCl3Ϊǿ�������Σ�Fe3��ˮ��Fe3����3H2OFe(OH)3��3H���������ԣ�pH��7��
(2)�ټ�ͬѧ��ΪFe3����3H2OFe(OH)3��3H��������Na2SO3��Һ��SO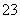 ���H��ʹc(H��)�½���ƽ�������ƶ������ɺ��ɫ��Fe(OH)3������ͬѧ��ΪFe3������SO
���H��ʹc(H��)�½���ƽ�������ƶ������ɺ��ɫ��Fe(OH)3������ͬѧ��ΪFe3������SO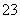 ������SO
������SO ��Fe2������Ӧ�����ӷ���ʽΪ2Fe3����SO
��Fe2������Ӧ�����ӷ���ʽΪ2Fe3����SO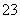 ��H2O===2Fe2����SO
��H2O===2Fe2����SO ��2H��
��2H��
(3)�����Ŀ������Ϣ����ͬѧ��Ϊī��ɫ��FeCl3���ɫ��FeSO3�Ļ����ҺҲ�����γɽ��壬���֡������ЧӦ����
(4)�ٸ�����ɫ��Ӧ������Fe2����K3[Fe(CN)6]��Һ��a��ȷ��b��c����Fe3�����������ԣ�SO2���л�ԭ�ԣ��ȷ���������ԭ��Ӧ������Fe2���ٲ���FeSO3��������Ӧ�����ӷ���ʽΪ2Fe3����SO2��2H2O===2Fe2����SO ��4H����Fe2����SO2��H2O===FeSO3����2H����FeSO3��FeCl3�Ļ��Һ�����ֺ��ɫ
��4H����Fe2����SO2��H2O===FeSO3����2H����FeSO3��FeCl3�Ļ��Һ�����ֺ��ɫ
�𰸡�(1)����(2)��Fe3����3H2OFe(OH)3��3H��������Na2SO3��c(H��)�½���ƽ�������ƶ�������Fe(OH)3����2Fe3����SO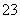 ��H2O===2Fe2����SO
��H2O===2Fe2����SO ��2H��
��2H��
(3)FeSO3��FeCl3�Ļ����ҺҲ���ܳ��֡������ЧӦ��
(4)��a����2Fe3����SO2��2H2O===2Fe2����SO ��4H����Fe2����SO2��H2O===FeSO3����2H����FeSO3��ʣ���FeCl3��Һ��϶����ֺ��ɫ
��4H����Fe2����SO2��H2O===FeSO3����2H����FeSO3��ʣ���FeCl3��Һ��϶����ֺ��ɫ


 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ӵ�����ˮ����ȡ���������ͼ��ʾ�������й�˵��������� (����)��

A��XΪSO2���壬Ҳ��ΪNa2SO3��Һ
B���豸YΪ������
C������ȡ��Ĺ�����һ����Br��������
D����ҵ��ÿ���1 mol Br2����Ҫ����Cl2��������Ϊ44.8 L(��״����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������Fe��FeO��Fe2O3��Fe3O4�Ļ�����м���150 mL 4 mol��L��1��ϡ����ǡ��ʹ�������ȫ�ܽ⣬�ų�2.24 L NO(��״����)����������Һ�м���KSCN��Һ����Ѫ��ɫ���֡�����������H2�ڼ��������»�ԭ��ͬ�����Ļ������õ����������ʵ���Ϊ (����)��
A��0.21 mol�� B��0.25 mol
C��0.3 mol�� D��0.35 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����仯��������ѧ��ѧ�е�һ����Ҫ���ʣ����й�����Ԫ�ص���������ȷ���� (����)��
A��2Fe3����Fe===3Fe2������˵�������ԣ�Fe3����Fe2��
B��25 �棬pH��0����Һ�У�Al3����NH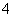 ��NO
��NO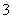 ��Fe2�����Դ�������
��Fe2�����Դ�������
C��5.6 g����������������Ӧʧȥ����Ϊ0.2 mol
D������������Һ�м������������Һ��Fe2����2H2O2��4H��===Fe3����4H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʵ���Ϊ0.10 mol��þ����ֻ����CO2��O2��������������ȼ��(���ﲻ��̼��þ)����Ӧ�������ڹ������ʵ�����������Ϊ (����)��
A��3.2 g�� B��4.0 g��
C��4.2 g�� D��4.6 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���Ҳ���MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ�Ʊ�MgAl2O4����Ҫ�������£�
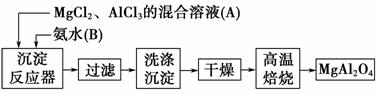
(1)ΪʹMg2����Al3��ͬʱ���ɳ�����Ӧ���������Ӧ���м���________(�A����B��)���ٵμ���һ��Ӧ�
(2)����ͼ��ʾ�����˲����е�һ��������________��
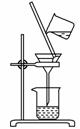
(3)�ж������г����Ƿ�ϴ�����õ��Լ���________�����±���ʱ������ʢ�Ź��������������________��
(4)��ˮAlCl3(183 ������)����ʪ��������������������ʵ���ҿ�������װ���Ʊ���

װ��B��ʢ�ű���NaCl��Һ����װ�õ���Ҫ������______________________ ________________________________________________________��
F���Լ���������_________________________________________________��
��һ������װ���ʵ��Լ���Ҳ����F��G�����ã���װ����Լ�Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һδ��ƽ�����ӷ���ʽΪ______��XO ��6H��===3X2��3H2O���ݴ��жϣ���������ͻ�ԭ��������ʵ���֮��Ϊ (����)��
��6H��===3X2��3H2O���ݴ��жϣ���������ͻ�ԭ��������ʵ���֮��Ϊ (����)��
A��1��1 B��3��1
C��5��1 D��7��1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com