
| A�������ձ���һ�����ᷢ�������ӷ�Ӧ�У�2Na��2H2O=2Na����2OH����H2�� |
| B�������ձ����ƾ���Һ���Ͼ��ҷ�Ӧ����ȶ��ԣ�X�ձ��еķ�Ӧƽ��Щ |
| C��Z�ձ���һ�����г������ɣ����������ǵ���ͭ |
| D�������ձ����û�������������ʵ���һ����ͬ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ͻ���۵�һ�����ֽ����� |
| B�����ƺϽ���Ͷ��һ������ˮ�п���ȫ�ܽ����ɫ��Һ����n(Al)��n(Na) |
| C�����ƺϽ�Ͷ�뵽�����Ȼ�ͭ��Һ�У�����������ͭ����Ҳ������ͭ���� |
| D����m g��ͬ��ɵ����ƺϽ�Ͷ�����������У��ų���H2Խ�࣬��������������ԽС |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��LiOH��ǿ�� |
| B��Be����ˮ���ҷ�Ӧ |
| C��Li�ڿ����в��ױ����� |
| D��Be(OH)2����NaOH��Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���۲����ʱ�Ƿ�ų����� |
| B���۲�μ�����ʱ�ܷ�ų����� |
| C������ˮ�У��ٵμ�ʯ��ˮ���۲����������� |
| D������ˮ�У��μ��������Ȼ�����Һ���۲����ް�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
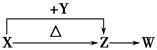
�鿴�𰸺ͽ���>>
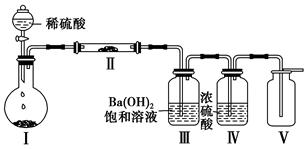
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
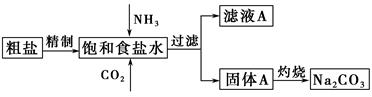
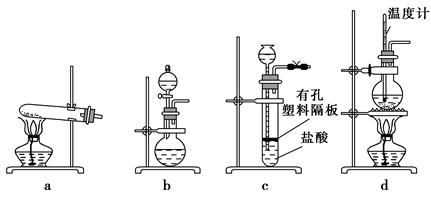
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������ŷŻ��������ЧӦ |
| B����ɫʳƷ�Dz����κλ�ѧ���ʵ�ʳƷ |
| C��Cu��ŨH2SO4��Ӧ��H2SO4�����������ԣ��ֱ������� |
| D���ü��ȵķ������ܼ���NaHCO3��Na2CO3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��NaOH��Na2CO3 | B��Na2CO3 |
| C��Na2CO3��NaHCO3 | D��NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
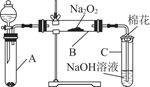
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡB�е�����������Ʒ���Թ��У��μ���������ˮ���ܽ⣬Ȼ��ȡ��������Һ�ֱ����ڢ��Թ��� | ������ȫ�ܽ� |
| ����2�������Թ��м��� ���ٵμ� | �� |
| ��֤���������к�Na2SO4 | |
| ����3�������Թ��� | |
| | �� �� |
| ��֤������������Na2SO3���� | |
| | |
| ��˵����������û��Na2SO3�� | |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com