

 NaAlO2+NH3��
NaAlO2+NH3��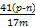 ��100%����ƫС����
��100%����ƫС����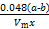 ��100%
��100%

 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| ���� | ʽ�� | ��״ | �ܶ�/g/cm3 | �е�/�� | �ܽ�� | |
| �� �� | 93 | ��ɫ��״Һ�壬 ���л�ԭ�� | 1.02 | 184.4 | ����ˮ | �������Ҵ������ѵ� |
| �� �� | 60 | ��ɫҺ�� | 1.05 | 118.1 | ������ˮ | �������Ҵ������� |
| �������� | 135 | ��ɫ���� | 1.22 | 304 | ������ˮ��������ˮ | �������Ҵ������� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
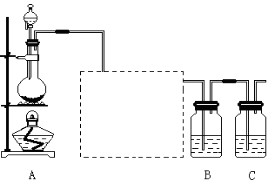
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | A | B | C | D |
| װ �� |  | 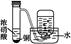 | 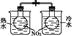 |  |
| ʵ �� | ���������Բ���п������ķ�Ӧ���� | �Ʊ����ռ�����NO���� | ��֤�¶ȶԻ�ѧƽ���Ӱ�� | �������ⸯʴʵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
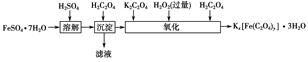
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
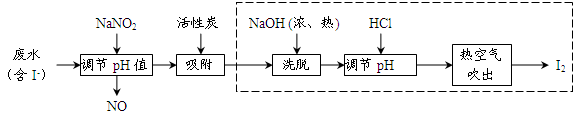
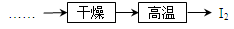
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������ʳ�üӵ����к��е� |
| B���ôס�ʯ��ˮ��֤�����к���̼���� |
| C���õ�Ƽ����������Ƿ��в������� |
| D���ü����ס�ʳ�Ρ�ˮ��ɵ����ʵ��ܽ⡢����ʵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
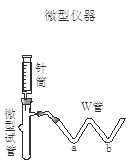
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ�����Թ��У���W��a������ ��b������ ���ý��ܽ�W�������Թ����Ӻ� | |
| ����2������Ͳ���� ������ͷ�������ԹܵĽ������������Ʒ��ע�����Һ�� | �� |
| ����3��������Ͳ����������ˮϴ���������� ע�����Թ��� | �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com