
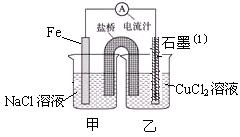
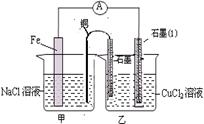
 NH3��H2O + H+��1�֣�
NH3��H2O + H+��1�֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ��� | ʵ����� | ʵ������ |
| I | ϡ�����м���ͭƬ | �����Ա仯 |
| II | ��������Һ�м���ͭƬ | �����Ա仯 |
| III | ϡ�����м�����������Һ | �����Ա仯 |
| �ټ���ͭƬ | ����ɫ���ݣ���Һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

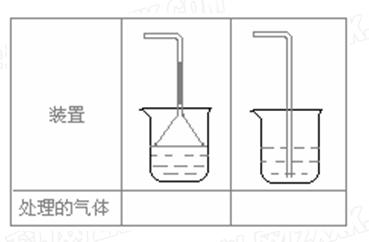
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽�� | Ԥ������ͽ��� | |
| �� | ȡ��������Ư�����Թ��У� | |
| �� | | |
| �� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
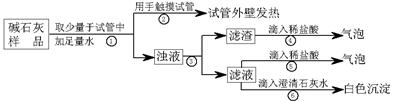
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��� | �缫���� | �������Һ | ����ָ��ƫת���� |
| 1 | Mg Al | ϡ���� | ƫ��Al |
| 2 | Al Cu | ϡ���� | ƫ��Cu |
| 3 | Al ʯī | ϡ���� | ƫ��ʯī |
| 4 | Mg Al | NaOH��Һ | ƫ��Mg |
| 5 | Al Zn | Ũ���� | ƫ��Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| | �Ƿ���ȷ | �������� |
| ѧ���Ĺ۵� | | |
| ѧ���ҵĹ۵� | | |
| ѧ�����Ĺ۵� | | |
A�� |
B�� |
C�� |
D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
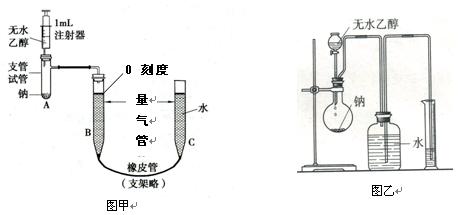
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
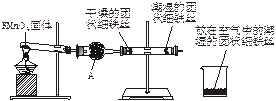
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com