
����������ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ���������������ȱѪ�����ʡ�������ij�о�С�������һ�������������H�ĺϳ�·�ߣ�
��1��ԭ��A��ͬ���칹���У����б����Һ˴Ź�����������5����Ľṹ��ʽΪ ��
д�������ʴ�������Ӧ�Ļ�ѧ����ʽ ��
��2���ڵķ�Ӧ������ ��ԭ��D�к��еĹ����������� �� ��
��3��д�����������������м����F��ͬ���칹��Ľṹ��ʽ ��
(i) �ܷ���������Ӧ��
(ii) �����к�����ȡ���ı����ṹ����������ȡ�����ǣ���COOCH3�� ���Ҷ��ߴ��ڶ�λ��
���Ҷ��ߴ��ڶ�λ��
��4��ԭ��B�������������������������ᣨHOOC-CH=CH-COOH�����������������ԭ��CH2OH-CH=CH-CH2OH�ϳ�������ĺϳ�·�ߡ��ϳ�·������ͼʾ�����£�

���������������1��ԭ��A�ķ���ʽΪC7H8O�����ĺ��б����Һ˴Ź�����������5�����ͬ���칹��ṹ��ʽΪ ��д�������ʴ�������Ӧ�Ļ�ѧ����ʽ
��д�������ʴ�������Ӧ�Ļ�ѧ����ʽ ����2����Ӧ�ڵĻ�ѧ��Ӧ����Ϊ������Ӧ����ȡ����Ӧ����ԭ��D�ڰ��������к��еĹ�����Ϊ�ǻ�����������3��F�ķ���Ҫ���ͬ���칹��
����2����Ӧ�ڵĻ�ѧ��Ӧ����Ϊ������Ӧ����ȡ����Ӧ����ԭ��D�ڰ��������к��еĹ�����Ϊ�ǻ�����������3��F�ķ���Ҫ���ͬ���칹�� ��
�� ��
�� ��
��
��4����ԭ��CH2OH-CH=CH-CH2OH�ϳ�������ĺϳ�·��Ϊ +HBr
+HBr
 ��
�� +O2
+O2 
 ��
�� +O2
+O2
 ��
�� +NaOH
+NaOH
 +NaBr+H2O��
+NaBr+H2O��
���㣺�����л�������š��ṹ��ʽ��ͬ���칹�����д���ϳɵ�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������F�����ɵ¹���ѧ�Һϳ�,Ŀǰ��������ըҩ��Ⱦ�ϵȡ���������ϳɹ���:
�ش���������:
(1)������E�ķ���ʽ����������,������F�ĺ˴Ź����������������������塣
(2)�ٵķ�Ӧ��������������,�ڵļ��ȷ�ʽͨ��������������,�ܵķ�Ӧ����������������
(3)д����Ӧ�Ļ�ѧ����ʽ: ����
(4)������G��B��ͬ���칹��,����������Ҫ��:����B������ͬ�Ĺ�����,�ڱ����ϵ�һԪȡ������ֻ������,��д��F�Ľṹ��ʽ��������������������������������
(5)��֪������D����������,�������ᷴӦ������,��д���÷�Ӧ�����ӷ���ʽ:�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���������Ĺ�ϵ�dz����У����ִ����ĸ��������л��ﶼ������Ҫ�����á������������ʣ����顢�����顢��ϩ����Ȼ�����������Ա����ӡ���ȩ��ţ�͡���ѡ����ȷ����������Ӧ�Ŀո��
��1�����ڱ��������� ��
��2�����ڷ����廯������� ��
��3�����ڻ������� ��
��4�������к���̼̼˫���������������� ��
��5����ϵͳ���������Ա����������� ��
��6����ȩ����������Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B���Ƿ����廯���1molAˮ��õ�1molB��1mol���ᡣA��B����Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����B������̼����Ԫ���ܵ���������Ϊ65��2%��A��Һ�������ԣ�����ʹFeCl3��Һ��ɫ��
��1��A��B����Է�������֮��Ϊ ��
��2��1��B������Ӧ���� ����ԭ�ӡ�
��3��A�������Ĺ������У� ��
��4��B���ܵĽṹ��ʽ�� ��
��5��1molB���ʷֱ���������̼��������Һ������������Һ��Ӧ���������Ķ��ߵ����ʵ���֮���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ŷ��л���������ά�IJ��������о��������ѿ�����һ�ֳ���������ά������ά��������PBO��ά�Ŀ������ܣ����һ�������PBO��ά�Ŀ�ѹ�����ܡ��������ԶԶ��ױ�Ϊԭ�Ϻϳɸ���ά�ĺϳ�·�ߣ���Щ��Ӧδע����������ش�
��1��д���ϳɸ���ά�ĵ���F�Ľṹ��ʽ�� ��
��2����Ӧ���ͣ�A��B ��B��C ��
��3��д����ѧ����ʽ��ע����Ӧ��������
B��C �� D��E ��
��4����1molF������Na2CO3��Һ��ȫ��Ӧ��������Na2CO3 mol
��5��д�����ַ��������������ʵĽṹ��ʽ�� �� ��
����B��Ϊͬ���칹�����FeCl3��Һ����ɫ��Ӧ
�ۺ˴Ź���������3��壬�ҷ����֮��Ϊ3��1��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Զ��ױ���Ӣ������p-xylene����дΪPX���ǻ�ѧ��ҵ����Ҫԭ�ϡ�
��1��д��PX�Ľṹ��ʽ ��
��2��PX���ܷ����ķ�Ӧ�� �� ���Ӧ���ͣ���
��3�����ܼ���DEHP����������ͼ��ʾ��ת����ϵ������A��PX��һ��ͬ���칹�塣
��B�ı����ϴ������ֲ�ͬ��ѧ��������ԭ�ӣ�C��������һ��̼ԭ�ӷֱ�����һ���һ���һ����������DEHP�Ľṹ��ʽ�� ��
��D�����еĹ������� �������ƣ���
��4��д��E��HBr��һ�������·�����Ӧ�Ļ�ѧ����ʽ����Ҫ����Ϊ�����к������϶�����ʣ��� ��
��5��F��B��һ��ͬ���칹�壬��������������
a.�DZ�����λ��ȡ����
b.��FeCl3��Һ��ʾ������ɫ
c.����̼��������Һ��Ӧ
д��F��NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���A����ͨ����ͬ�ķ�Ӧ�õ��������ʣ�
��1��A�Ľṹ��ʽ��_________________
��2��A��_______________����д��ѧʽ����Ӧ����D
��3���л���C�й����̼ԭ�������_______________��
��4�������廯����F��A��ͬ���칹�壬1molF���Ժ�3mol NaOH�����кͷ�Ӧ��F�����ϵ�һ�ȴ���ֻ��һ�֣��˴Ź���������ʾF�����������ֲ�ͬ����ԭ�ӡ�д��F���ֿ��ܵĽṹ��ʽ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Է�������Ϊ92��ij������X��һ����Ҫ���л�����ԭ�ϣ��о���������Ϊ��ʼԭ����Ƴ���ͼ��ʾ�Ĺ�ϵͼ(���ֲ����Ӧ������ȥ)������A��һ�ȴ��H��һ�ֹ��ܸ߷��ӣ��������ΪC7H5NO��
��֪��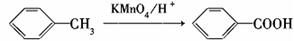
�������ѧ֪ʶ�뱾��������Ϣ�ش��������⣺
��1��H�Ľṹ��ʽ��________________��
��2����Ӧ�ڢ۵ķ�Ӧ���ͷֱ���________________ ��
��3����Ӧ�ݵĻ�ѧ����ʽ�� ��
��4�� �ж���ͬ���칹�壬���к���1��ȩ����2���ǻ��ķ����廯����,��˴Ź�������ͼ�з����֮��Ϊ1:2:2:1���������������������ͬ���칹��Ľṹ��ʽ�� ��
�ж���ͬ���칹�壬���к���1��ȩ����2���ǻ��ķ����廯����,��˴Ź�������ͼ�з����֮��Ϊ1:2:2:1���������������������ͬ���칹��Ľṹ��ʽ�� ��
��5�����úϳɷ�Ӧ����ͼ��ʾ���� ����������ϳ�
����������ϳ� ��������ķ�����������4���������磺
��������ķ�����������4���������磺
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������A(����ʽΪC6H6O)��һ���л�����ԭ�ϣ��ڿ������ױ�������A���й�ת����Ӧ����(���ַ�Ӧ������ȥ)��
��֪���� 
�� (R��ʾ������R���R���ʾ��������)
(R��ʾ������R���R���ʾ��������)
��1��д��A�Ľṹ��ʽ�� ��
��2��G�dz���ָʾ����̪��д��G�к��������ŵ����ƣ� �� ��
��3��ij��������E��ͬ���칹�壬�ҷ�����ֻ�����ֲ�ͬ��ѧ�������⡣д���û�����Ľṹ��ʽ�� �� �������֣�
��4��F��D��Ϊͬ���칹�塣д����ӦE��F�Ļ�ѧ����ʽ ��
��5����������֪ʶ����������Ϣ��д����A��HCHOΪԭ���Ʊ� �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�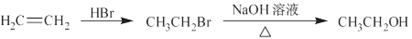
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com