
��һ���¶��£���һ���ݻ�����������У�ͨ��2 mol A2��8 mol B2�����������ʹ֮��Ӧ����֪��A2(g)��3B2(g)
 2AB3(g)����H����Q kJ��mol��1��ƽ��ʱ����������ѹǿΪ��ʼʱ��80%��
2AB3(g)����H����Q kJ��mol��1��ƽ��ʱ����������ѹǿΪ��ʼʱ��80%��
(1)ƽ��ʱ������AB3���������Ϊ________��
(2)����2 mol A2��8 mol B2����ƽ��ʱ���ų�����Ϊ________(�����)��
A����Q kJ B������Q kJ
C������Q kJ D�����ܴ��ڻ�С�ڻ����Q kJ
(3)����ͬ�������У������¶ȣ�ͨ��2 mol AB3��1 mol B2�������������Ӧ��ƽ��ʱ��AB3����������������ж���ȷ����________(�����)��
A��һ������1/4 B��һ������1/4
C��һ��С��1/4 D�����ܴ��ڻ�С�ڻ����1/4
������_________________________________________________________________��
(4)����ͬһ�¶ȣ�����ͬ�����У���ʼͨ��һ�����ʵ�����A2��B2��AB3����ʹƽ��ʱAB3���������Ϊ1/4������ʼʱ��Ӧ����Ϊ������Ӧ������У������A2�����ʵ���a mol��ȡֵ��Χ��_______________________________________________________________��
�𰸡�(1)25%��(2)B
(3)D����ʼͨ��2 mol NH3��1 mol H2�൱��1 mol N2��4 mol H2���������յ�ƽ��״̬�൱�����е�ƽ��״̬���м�ѹ���¡���ѹ��NH3�����������С�����£�NH3�����������������ѡD
(4)1<a��2
������(1)A2(g)��3B2(g)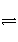
 2AB3(g)
2AB3(g)
����ʼ����2 mol�� 8 mol����0
��ת������x������ 3x��������2x
��ƽ�⣺��2��x��8��3x���� 2x
��ƽ��ʱ����������ѹǿΪ��ʼʱ��80%���� ��100%��80%��x��1 mol����AB3���������Ϊ��
��100%��80%��x��1 mol����AB3�����������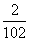 ��100%��25%��
��100%��25%��
(2)����ƽ��ʱ����AB3�����ʵ���Ϊ2 mol�����Էų�������ΪQ kJ����ѡ��B�������⡣
(3)��ʼͨ��2 mol AB3��1 mol B2�൱��1 mol A2��4 mol B2���������յ�ƽ��״̬�൱�����е�ƽ��״̬���м�ѹ���¡���ѹ��AB3�����������С�����£�AB3�����������������D����ȷ��
(4)��Ϊ��ƽ���������ƽ���Ч������ʼʱAB3�����ʵ���Ϊ0����A2ӦΪ2 mol������ʼʱAB3����ƽ��ʱ��2 mol����A2Ϊ1 mol������ʼ��Ӧ����Ϊ������Ӧ������У���A2�����ʵ���ȡֵ��Χ��1<a��2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪������KMnO4����μ�Ũ���ᣬ��������ɫ���壻
����FeCl2��Һ��ͨ������ʵ��ٲ��������壬��Һ���ɫ��
��ȡʵ������ɵ���Һ���ڵ���KI��ֽ�ϣ���ֽ����ɫ��
�����ж���ȷ����
A������ʵ��֤�������ԣ�MnO4����Cl2��Fe3����I2 B������ʵ���У���������������ԭ��Ӧ
C��ʵ������ɵ����岻��ʹʪ��ĵ���KI��ֽ���� D��ʵ���֤��Fe2���������������л�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��100mL 0.1 mol·L��1�������[NH4Al��SO4��2]��Һ����ε���0.1 mol·L��1 Ba��OH��2��Һ������Ba��OH��2��Һ���V�ı仯�����������ʵ���n�ı仯����ͼ��ʾ��������˵������ȷ���ǣ� ��
A��c����Һ�ʼ���
B��b�㷢����Ӧ�����ӷ���ʽ�ǣ�Al3++2SO42-+2Ba2++3OH— =
Al��OH��3��+2BaSO4��
C��c�����Ba��OH��2��Һ�����Ϊ200 mL
D��a�����Һ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������ԭ�����͵���(����)
A�����¼������������ʹ�ϳɰ��ķ�Ӧ���ʼӿ�
B���غ�ɫNO2��ѹ����ɫ�ȱ�����dz
C��SO2��������SO3�ķ�Ӧ��������Ҫʹ�ô���
D��H2��I2��HIƽ��������ѹ����ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ȫ�ӳɺ���������2,2,3�������������(����)
A��HC��CCH(CH3)C(CH3)3
B��CH2===CHC(CH3)2CH(CH3)2
C��(CH3)3CC(CH3)===CHCH3
D��(CH3)3CCH(CH3)CH===CH2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ں��¡����ݵ��ܱ������з�����ӦA(g)
 B(g)��C(g)������Ӧ���Ũ����2 mol/L����0.8 mol/L��20 s����ô��Ӧ��Ũ����0.8 mol/L����0.2 mol/L�����ʱ��Ϊ
B(g)��C(g)������Ӧ���Ũ����2 mol/L����0.8 mol/L��20 s����ô��Ӧ��Ũ����0.8 mol/L����0.2 mol/L�����ʱ��Ϊ
(����)��
A��10 s B������10 s
C��С��10 s D�����ж�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E�ֱ�������ֶ�����Ԫ�أ���ԭ����������������֪��B�����������Ų���ns2npn��1��C��p�ܼ���δ�ɶԵĵ��ӱ�B��һ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��E��Dͬ������E�ڸ�������ԭ�Ӱ뾶��С��B��A�ĵ��������ɾ��д̼�����ζ�����塣
(1)Bԭ�Ӻ�������Ų�ʽΪ________��
(2)A��E����ʱԭ�Ӽ���________�����ϣ�D��C����ʱԭ�Ӽ���
________�����ϡ�
(3)д��A��C�ĵ���ֱ�ӻ����γɵĻ�������E���ʷ�Ӧ�����ӷ���ʽ��
__________________________________________________________________
_________________________________________________________________��
(4)A��B�γɻ�����ʱ������ԭ�Ӳ�ȡ________�ӻ��ɼ���������ṹΪ
________������________(����ԡ��Ǽ��ԡ�)���ӡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com