
| A�� | ��Ҫ����NaClO���������Ϊ144.0 g | |
| B�� | ��ͼ��ʾ�������У��������Dz���Ҫ�ģ������һ�ֲ�������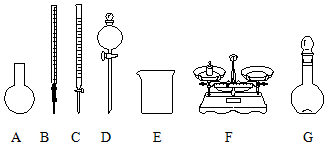 | |
| C�� | ���Ƶ���Һ�ڿ����й��գ����ú���Һ��NaClO�����ʵ���Ũ�ȼ�С | |
| D�� | ����ƿ������ˮϴ����Ӧ��ɲ���������Һ���ƣ�������ƫ�� |
���� A������ƿ�Ĺ��û��480mL��Ӧѡȡ500 mL������ƿ�������ƣ�
B����Ҫ���������衢������������Ҫ��ͷ�ιܣ�
C������NaClO�����տ����е�H2O��CO2�����ʣ�����NaClO����Һ���ʵ���NaClO���٣�
D������ƿ����ˮ������Һ�������Ӱ�죮
��� �⣺A��Ӧѡȡ500 mL������ƿ�������ƣ�Ȼ��ȡ��480mL���ɣ�������ҪNaClO��������500mL��1.2 g•cm-3��25%=150.0g����A����
B������������ƽ����NaClO���壬�����ձ����ܽ�NaClO�����ò��������н������������������ƿ�ͽ�ͷ�ι������ݣ�ͼʾ��A��B��C��D����Ҫ�������貣�����ͽ�ͷ�ιܣ���B����
C������NaClO�����տ����е�H2O��CO2�����ʣ�����NaClO����Һ���ʵ���NaClO���٣����Ƶ���Һ�����ʵ����ʵ�����С������Һ�����ʵ���Ũ��ƫС����C��ȷ��
D������ƿ����ˮ������Һ�������Ӱ�죬���Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ�ã���D����
��ѡC��
���� ���⿼����һ�����ʵ���Ũ����Һ���ƵIJ��衢�����Լ��������ȣ��ѶȲ��Ǻܴ�ע��������ʵ�����ʱ����Һ�������500mL���㣮


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���ȷ�ʽ | ����Ԫ����� | ��Ԫ�ص���������/% | |
| Fe | O | ||
| �ƾ��� | Fe��O | 74.50 | 25.50 |
| �����־ƾ��� | Fe��O | 76.48 | 23.52 |
| �ƾ���� | Fe | 100.00 | 0.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ҽ����̼���ơ�Al��OH��3������������θ����� | |
| B�� | ���������۵�ܸߣ����������ͻ���ϣ�����Ҫ�ɷ���SiO2 | |
| C�� | ˮ��������������ճ�ϼ��ͷ���� | |
| D�� | ����ˮ�м�������������ˮ������Al��OH��3���������ɱ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢܢ� | B�� | �ۢ� | C�� | �ڢܢ� | D�� | �ܢ٢� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com