
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸�� A �оƾ�������)��

(1)ʵ���������������ӷ���ʽΪ_____��
(2)װ�� B �б���ʳ��ˮ��������_____����д��װ�� B ����һ������_______________________________��
(3)װ�� C ��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ� Ϊ�� C ���������������η������ʵ������_______________(����)��
��� | a | b | c | d |
�� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
(4)���װ�� D��E ��Ŀ���DZȽ��ȡ��塢��ķǽ���������Ӧһ��ʱ���������װ�� D ��������Һ����װ�� E ���������������۲쵽��������_____����������˵����ķǽ�����ǿ�ڵ⣬ԭ����_________��
(5)װ�� F �������������ӷ�Ӧ����ʽ��_____��
���𰸡�MnO2 + 2Cl-+ 4H+=Mn2++ Cl2��+ 2H2O ��ȥ�����е��Ȼ��� ���ʵ�����ʱ C ���Ƿ������� d ��Һ�ֲ㣬�ϲ�Ϊ�Ϻ�ɫ���²�ӽ���ɫ ����������ʱ��Ҳ�����û����ⵥ�� Cl2 + SO32- + H2O====2Cl- + SO42- + 2H+
��������
��1��ʵ���������������ӷ���ʽΪMnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
��2��װ��B�б���ʳ��ˮ�������dz�ȥ�����е��Ȼ��⣬���ʵ�������װ��C������������װ��B�г���©���е�Һ��ͻ�������
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư������
��4���ȵ��ʺ��廯�Ʒ�Ӧ�����嵥�ʣ��嵥�ʺ͵⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ�����ڱ����Ϻ�ɫ�����۲쵽�������ǣ�E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ���²�ӽ���ɫ����������ǿ�����ԣ������������ܹ��������������ɵⵥ�ʣ����Ը�������˵����ķǽ�����ǿ�ڵ���
��5��װ��F�����������ն������������ֹ��Ⱦ��������Ӧ�����ӷ���ʽΪ��Cl2+SO32-+H2O=2Cl- +SO42-+2H+��
��1��װ��A�еĹ���ҩƷ��MnO2����Һ©����ʢ�ŵ���Ũ���ᣬMnO2��Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ��4HCl+MnO2![]() MnCl2+Cl2��+2H2O�����ӷ���ʽ��MnO2+4H++2Cl-
MnCl2+Cl2��+2H2O�����ӷ���ʽ��MnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+4H++2Cl-
Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
��2��װ��B�б���ʳ��ˮ�������dz�ȥ�����е��Ȼ��⣬���ʵ�������װ��C������������װ��B�г���©���е�Һ��ͻ�����������װ��B�����ü��ܳ�ȥ�����е��Ȼ��⣬���ܼ��ʵ�����ʱC���Ƿ����������ʴ�Ϊ����ȥ�����е��Ȼ��⣬���ʵ�����ʱC���Ƿ���������
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ�I����ʪ�����ɫ��������II��Ӧ�����̬������Ҳ�������������III�����ø������ɫ����������C��I��II��III���η���ʪ�����ɫ��������ˮ�Ȼ��ơ��������ɫ��������ѡd���ʴ�Ϊ��d��
��4����������װ��D��������Һ����װ��E�У��ȵ��ʺ��廯�Ʒ�Ӧ�����嵥�ʣ��嵥�ʺ͵⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ�����ڱ����Ϻ�ɫ�����۲쵽�������ǣ�E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ���²�ӽ���ɫ����������ǿ�����ԣ������������ܹ��������������ɵⵥ�ʣ����Ը�������˵����ķǽ�����ǿ�ڵ⣻
�ʴ�Ϊ����Һ�ֲ㣬�ϲ�Ϊ�Ϻ�ɫ���²�ӽ���ɫ������������ʱ��Ҳ�����û����ⵥ����
��5��װ��F�����������ն������������ֹ��Ⱦ��������Ӧ�����ӷ���ʽΪ��Cl2+SO32-+H2O=2Cl- +SO42-+2H+���ʴ�Ϊ��Cl2+SO32-+H2O=2Cl- +SO42-+2H+��


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��������������ԭ�����͵���
A. �ϳɰ���ҵ������ѹǿ����߷�Ӧ���ת����
B. ����ɫ����ˮ���պ���ɫ��dz
C. ��H2��I2������HI��ɵ�ƽ����ϵ��ѹ����ɫ����
D. �ں���Fe(SCN)3�ĺ�ɫ��Һ�м����ۣ����ã���Һ��ɫ��dz����ȥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����᳧��������Ҫ�ɷ���Fe2O3����κ�������SiO2��FeS����ҵ���������᳧���������մ����Ƶ��̷�(FeSO4��7H2O)��һ�־�������[Fe(OH)SO4]n���̷�������ȱ����ƶѪҩƷ����Ҫ�ɷ�����������������Ҫ��ˮ���������������������������£�
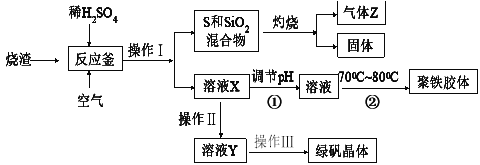
��ͨ�������ش�����������⣺
��1�����ղ�����β���Դ�������Ⱦ����˹�ҵ�����б�����л��մ��������з������е���_______________��
A���ð�ˮ���� B. ֱ����ˮ����
C. ��Ũ�������� D. ����������ʯ��ʯ��ĩ����Һ����
��2����������_____________��_______________�����ˡ�ϴ�ӡ����ﱣ��ȣ�
��3��������ҺX�ƾ������������������pH���������¶�Ŀ����___________________��
��4���ڷ�Ӧ����FeS��O2��ϡH2SO4��Ӧ�����������ʣ���Ӧ�����ӷ���ʽΪ___________________________��
��5��ij�о���С��Ϊ̽���̷�(��Է���������278)�ڸ������������¼��ȷֽⷴӦ�IJ���(��֪�ֽ����ȫΪ������)����������ͼ��ʾ��ʵ�飺
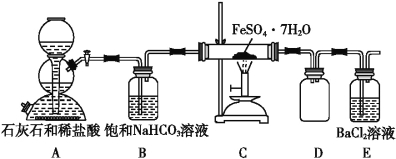
�� ʵ�鿪ʼʱ���ڵ�ȼC���ƾ����֮ǰӦ�ȴ���ͨCO2����Ŀ����_____________�� װ��D��������______________________��
������ͬѧ��Ƶ�װ�ô�����һ�����Ե�ȱ����____________________________��
������ͬѧ��ȡ55.6 g�̷�����ͼװ�ý���ʵ�顣���̷��ֽ���ȫ����Eƿ�в�����23.3 g��ɫ��������C�й������Ͷ������ϡ��������ȫ�ܽ�õ���Һ��ȡ��������Һ����KSCN��Һ�ʺ�ɫ����ȡ��������Һ��������KMnO4��Һ��KMnO4��Һ����ɫ����д���̷��ֽ�Ļ�ѧ����ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������0.1molCH3COONa��0.05molHCl����ˮ���1L��Һ(pH��7).
��1�������ӷ���ʽ��ʾ����Һ�д��ڵ�����ƽ����ϵ______________��_______________��___________________
��2����Һ�и����ӵ����ʵ���Ũ���ɴ�С˳��Ϊ_____________________________________________________
��3����Һ��������Ũ��Ϊ0.1mol/L����________________��Ũ��Ϊ0.05mol/L����____________________
��4�����ʵ���֮��Ϊ0.lmol������������______________��__________
��5��CH3COO-��OH-�����ʵ���֮�ͱ�H+��________mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
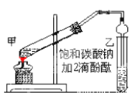
����Ŀ��ij��ѧѧϰС���������װ�ã���Ũ������ľ̿�ķ�Ӧ����̽����

��ش��������⣺
(1)����װ�������Ժ�ȼ�ճ��е�ľ̿�ھƾ����ϼ���������״̬������������ƿ�У�������ƿ���� �μ�Ũ���ᡣ���Թ۲쵽������ƿ���������ɫΪ______������������Ļ�ѧ����ʽ��______��
(2)װ�� C ��ʢ��������Ũ Ba(OH)2 ��Һ����Ӧһ��ʱ���ɹ۲쵽 C �г��ְ�ɫ�������ð�ɫ����Ϊ__(д��ѧʽ)��
(3)װ�� B ��������______��
(4)װ�� D ���ռ�������ɫ���壬����ͬѧ��Ϊ�� NO������ͬѧ��Ϊ�� O2�����жԸ�����ļ��鷽�����ʵ���_____(����ĸ����)��
A.���ڹ۲�װ�� D �м���ƿ���������ɫ�仯
B.��ʪ�����ɫʯ����ֽ���뼯��ƿ�ڣ��۲�ʯ����ֽ�Ƿ���
C.�����ǵ�ľ�����뼯��ƿ�ڣ��۲�ľ���Ƿ�ȼ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ������ȡ������������Ҫ�������£�
���ڼ��Թ�(��ͼ)�м���2mLŨ���ᡢ3mL�Ҵ���2mL����Ļ����Һ��
�ڰ���ͼ���Ӻ�װ��(װ������������)��������Һ��С����ȵؼ���3��5min��
�۴��Թ����ռ���һ���������ֹͣ���ȣ������Թܲ�������Ȼ���ô��ֲ㡣
�ܷ�������������㡢ϴ�ӡ����
(1) д���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�_______________________��
(2) ��̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ���������____________��
(3)���Թ��У������Һ����ȷ����˳��________________________��
(4)���������Ҫ��С����ȼ��ȣ�����Ҫԭ����____________________________________��
(5)����ʵ���б���̼������Һ��������____________(����ĸ����)��
A. ��Ӧ��������Ҵ� B. ��Ӧ�����Ტ�����Ҵ�
C. ������������ D. �����������ɣ���������
(6)�������Թ��е����ʷ��뿪�Եõ���������������ʹ�õ�������____________������ʱ����������Ӧ�ô�����____________(����¿ڷ��������Ͽڵ���)����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϣ�����Fe2(SO4)3��Һ����ʴҺ����ʴͭ�ʵ�·��õ���Һ��Ҫ�ɷ���FeSO4��CuSO4��������Fe2(SO4)3��ijС�����װ�ôӷ�Һ����ȡͭ����ͼ��
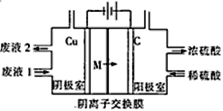
��֪��Fe2+ʧ����������OH��ǿ������˵����ȷ����
A. ����������û��ͭ������ԭ����2H+ + 2e�� = H2��
B. ʯī���ĵ缫��ӦʽΪ2H2O + 4e�� = 4H+ + O2��
C. ������Һ2����������ʱ��������ʴҺ����������Һ��
D. ����·��ת��2mol���ӣ���������2molM�ӽ���Ĥ������Ҳ�Ǩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ�������������
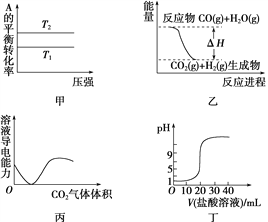
A. ��ͼ�����жϣ����ڷ�ӦA(g)��B(g) ![]() 2C(g)����T1>T2������H<0
2C(g)����T1>T2������H<0
B. ͼ�ұ�ʾ���淴ӦCO(g)��H2O(g) ![]() CO2(g)��H2(g)����H>0
CO2(g)��H2(g)����H>0
C. ͼ����ʾCO2ͨ�뱥��Na2CO3��Һ�У���Һ�����Ա仯
D. ͼ����ʾ0.1 mol��L��1������ζ�20 mL 0.1 mol��L��1 NaOH��Һ����ҺpH�������������ı仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W���ֻ�������ɶ�����Ԫ����ɡ�����X��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��WΪ��ɫ��ζ���塣�����ֻ������������ͼת����ϵ(���ַ�Ӧ����P��Ӧ��������ȥ)����ش�

(1)Y�ĵ���ʽ��____________________��
(2)X��Y����Һ�з�Ӧ�����ӷ���ʽ��__________________________________________��
(3)X���е�����Ԫ��֮��(���֡����ֻ�����)����ɶ��ֻ����ѡ������ijЩ�����������ͼװ��(�г̶ֹ�װ������ȥ)����ʵ�飬װ�â��в�����ɫ������װ�â��п��ռ���һ����ɫ���塣

��װ�â��з�Ӧ�Ļ�ѧ����ʽ��_________________________________________________��װ�â��з�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
����X���е�����Ԫ���е�������ɵ�ij������ڴ����������Ʊ����ռ����������װ�â������壬�û�����Ļ�ѧʽ��___________����������װ����_______________�� (����ͼѡ���Ҫװ�ã���д���)��
(4)��Z��Һ��ͨ�����������Ƶ�ij�������������г��õ�Ư�ס����������ʣ�ͬʱ��X���ɣ��÷�Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com