

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| 18a |
| b |
| 18a |
| b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
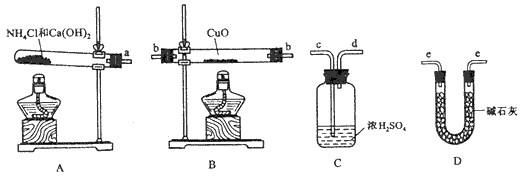
(11��)���ݰ�����ԭ����ͭ�ķ�Ӧ������ƲⶨͭԪ�����ԭ������ô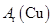 (����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H20)���ɴ˼���
(����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H20)���ɴ˼���![]() ��Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4C1��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��
��Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4C1��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��
��ش��������⣺
(1)������ԭ��������ͭ�Ļ�ѧ����ʽΪ________________________________��
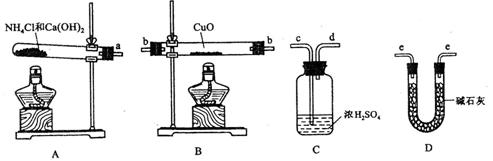
(2)�����ṩ���������Լ���ѡ����װ��ʵ���һ��������װ�ã����������������˳��Ϊ(��ͼ�б�ע�ĵ��ܿڷ��ű�ʾ)a��______________________________��
(3)�ڱ�ʵ���У������m(CuO)= a g��m(H2O)= b g����Ar(Cu)= _______________��
(4)�ڱ�ʵ���У�ʹ�ⶨ���Ar(Cu)ƫ�����_______________ (�����)��
1Cu0δ��ȫ��Ӧ �� CuO������
3Cu0�л��в���Ӧ������ �ܼ�ʯ�Ҳ�����
��NH4C1��Ca(OH)2����ﲻ����
(5)�ڱ�ʵ���У�����ͨ���ⶨ_______________��_______________����_______________��_______________�ﵽʵ��Ŀ�ġ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��߿���ѧ�������ר��ʮ�� ��ѧʵ�� ���ͣ�ʵ����
���ݰ�����ԭ����ͭ�ķ�Ӧ������ƲⶨͭԪ�����ԭ������ô (����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������
(����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������ m(H20)���ɴ˼���
m(H20)���ɴ˼��� ��Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4C1��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��
��Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4C1��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��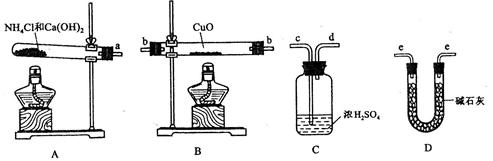
��ش��������⣺
(1)������ԭ��������ͭ�Ļ�ѧ����ʽΪ________________________________��
(2)�����ṩ���������Լ���ѡ����װ��ʵ���һ��������װ�ã����������������˳��Ϊ(��ͼ�б�ע�ĵ��ܿڷ��ű�ʾ)a��______________________________��
(3)�ڱ�ʵ���У������m(CuO)=" a" g��m(H2O)= b g����Ar(Cu)= _______________��
(4)�ڱ�ʵ���У�ʹ�ⶨ���Ar(Cu)ƫ�� ����_______________ (�����)��
����_______________ (�����)��
1Cu0δ��ȫ��Ӧ �� CuO�� ����
����
3Cu0�л��в���Ӧ������ �ܼ�ʯ�Ҳ�����
��NH4C1��Ca(OH)2����ﲻ����
(5)�ڱ�ʵ���У�����ͨ���ⶨ_______________��_______________����__________ _____��_______________�ﵽʵ��Ŀ�ġ�
_____��_______________�ﵽʵ��Ŀ�ġ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010����ͨ�ߵ�ѧУ����ȫ��ͳһ���Ի�ѧ���⣨���Ͼ��� ���ͣ�ʵ����
(11��)���ݰ�����ԭ����ͭ�ķ�Ӧ������ƲⶨͭԪ�����ԭ������ô (����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H20)���ɴ˼���
(����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H20)���ɴ˼���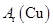 ��Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4C1��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��
��Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4C1��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��
��ش��������⣺[��Դ:ѧ��ơ���Z��X��X��K]
(1)������ԭ��������ͭ�Ļ�ѧ����ʽΪ________________________________��
(2)�����ṩ���������Լ���ѡ����װ��ʵ���һ��������װ�ã����������������˳��Ϊ(��ͼ�б�ע�ĵ��ܿڷ��ű�ʾ)a��______________________________��
(3)�ڱ�ʵ���У������m(CuO)= a g��m(H2O)= b g����Ar(Cu)= _______________��
(4)�ڱ�ʵ���У�ʹ�ⶨ���Ar(Cu)ƫ�����_______________ (�����)��
1Cu0δ��ȫ��Ӧ �� CuO������
3Cu0�л��в���Ӧ������ �� ��ʯ�Ҳ�����
��NH4C1��Ca(OH)2����ﲻ����
(5)�ڱ�ʵ���У�����ͨ���ⶨ_______________��_______________����_______________��_______________�ﵽʵ��Ŀ�ġ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɶ����и�һ��ѧ����ĩ���Ի�ѧ ���ͣ�ʵ����
���ݰ�����ԭ����ͭ�ķ�Ӧ������ƲⶨͭԪ�����ԭ������Ar(Cu)(����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������m(H2O)��ͬʱ������һ�ֶԻ����������壬�ɴ˼���Ar(Cu)��Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4C1��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��[��Դ:ѧ_��_��Z_X_X_K]

��ش��������⣺
(1)����װ��A�������Եķ�����
��
(2)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��������ԭ��������ͭ�Ļ�ѧ����ʽΪ ��
(3)�����ṩ���������Լ���ѡ����װ��ʵ���һ��������װ�ã����������������˳��Ϊ(��ͼ�б�ע�ĵ��ܿڷ��ű�ʾ)a�� ��
(4)�ڱ�ʵ���У������m(CuO)=ag��m(H2O)=bg����Ar(Cu)= ��
(5)�ڱ�ʵ���У�ʹ�ⶨ���Ar(Cu)ƫ�����_______________(�����)��
��CuOδ��ȫ��Ӧ ��CuO������ ��CuO�л��в���Ӧ������
�ܼ�ʯ�Ҳ����� ��NH4C1��Ca(OH)2����ﲻ����
(6)�ڱ�ʵ���У�����ͨ���ⶨ______________��_____________����_______________��_______________�ﵽʵ��Ŀ�ġ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com