
ij�����з�����һ����ѧ��Ӧ����Ӧ�����д���H2O��ClO����CN����HCO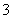 ��N2��Cl���������ӣ��ڷ�Ӧ�����в��ClO����N2�����ʵ�����ʱ��仯��������ͼ��ʾ�������ж�����ȷ����(˫ѡ)(����)
��N2��Cl���������ӣ��ڷ�Ӧ�����в��ClO����N2�����ʵ�����ʱ��仯��������ͼ��ʾ�������ж�����ȷ����(˫ѡ)(����)
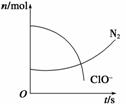
A����ԭ����CN������������ֻ��N2
B����������ClO������ԭ������HCO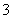
C����ƽ���������뻹ԭ���Ļ�ѧ������֮��Ϊ5��2
D����״����������2.24 L N2����ת��1 mol����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڻ�ѧ��ʴ��绯ѧ��ʴ��˵���У��������
(����)��
A�����DZ���ʴ�Ľ���ʧȥ���ӵĹ���
B��������Ҫ�������μӵķ�Ӧ
C����ѧ��ʴ��绯ѧ��ʴ������ͬʱ������
D������ˮ�����еĸ�ʴһ�㲻���ڵ绯ѧ��ʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��һ�����淴Ӧ��������ӦΪ�Է����̣������淴ӦΪ���Է����̣���֮����Ȼ��
(1)��֪2CO(g)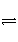
 CO2(g)��C(s)��T��980 Kʱ��H��T��S��0������ϵ�¶ȵ���980 Kʱ�����Ʀ�H��T��S____0(����ڡ�����С�ڡ����ڡ�����ͬ)������ϵ�¶ȸ���980 Kʱ�����Ʀ�H��T��S____0��
CO2(g)��C(s)��T��980 Kʱ��H��T��S��0������ϵ�¶ȵ���980 Kʱ�����Ʀ�H��T��S____0(����ڡ�����С�ڡ����ڡ�����ͬ)������ϵ�¶ȸ���980 Kʱ�����Ʀ�H��T��S____0��
(2)���ӹ�ҵ����ϴ��Ƭ�ϵ�SiO2(s)�ķ�ӦΪ
SiO2(s)��4HF(g)===SiF4(g)��2H2O(g)
��H(298.15 K)����94.0 kJ��mol��1
��S(298.15 K)����75.8 J��mol��1��K��1��
�覤H�ͦ�S�����¶ȶ��仯����˷�Ӧ�Է����е��¶���________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������м������ӷ�Ӧ��
��Cr2O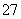 ��14H����6Cl��===2Cr3����3Cl2����7H2O
��14H����6Cl��===2Cr3����3Cl2����7H2O
��2Fe2����Br2===2Fe3����2Br��
��2Fe3����SO2��2H2O===2Fe2����SO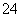 ��4H��
��4H��
�����й����ʵıȽ�����ȷ����(����)
A�������ԣ�Cr2O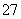 >Cl2>Fe3��
>Cl2>Fe3��
B�������ԣ�Cl2>Br2>Cr2O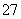
C����ԭ�ԣ�SO2<Fe2��<Br��
D����ԭ�ԣ�Cl��>Cr3��>Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
H3PO2�Ĺ�ҵ�Ʒ��ǣ�������(P4)��Ba(OH)2��Һ��Ӧ����PH3�����Ba(H2PO2)2����������H2SO4��Ӧ��д��������Ba(OH)2��Һ��Ӧ�Ļ�ѧ����ʽ________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʢ��10 mL 1.0 mol·L��1�ĵ�ˮ���Թ��У�ͨ������Cl2��ȫ��Ӧ��ת����0.1 mol���ӡ�д���÷�Ӧ�Ļ�ѧ����ʽ��______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����(1 100��)�����ܱ������з�����Ӧ��Na2SO4(s)+4H2(g)  Na2S(s)+
Na2S(s)+
4H2O(l)������˵������ȷ����( )
A.��ƽ�ⳣ������ʽΪ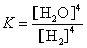
B.�����������������ѹǿ���ֲ��䣬����˵���÷�Ӧ�Ѵﵽƽ��״̬
C.��Na2SO4�������ı���ʼ����H2��Ũ�ȣ���ﵽƽ��ʱ��H2ת���ʲ���
D.����ʼʱͶ��2.84 g Na2SO4��һ����H2����Ӧ�ﵽƽ��ʱ�����ڹ��干��2.264 g����Na2SO4��ת����Ϊ25%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2HCHO+NaOH(Ũ) CH3OH+HCOONa ��Ӧ�У���ȩ�����ķ�Ӧ��( )
CH3OH+HCOONa ��Ӧ�У���ȩ�����ķ�Ӧ��( )
A.�������� B.������ԭ
C.�ȱ������ֱ���ԭ D.��δ��������δ����ԭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ��������ġ��������䡱���̣�
(1)���еġ�������ָ________����
(2)����Ҫ�ɷֵķ���ʽ��________������ʽΪ____________________________________��
�ṹʽΪ________��
(3)�������ԭ�ӵĿռ�ֲ���________�ṹ��
(4)���ʺ��ڱ�ʾ���ӿռ乹�͵�ģ����______________________________________��
(5)����Ҫ�ɷ���Cl2�ڹ��������µķ�Ӧ����________(�Ӧ����)���������ɵ��л�����ֱ���__________��________��________��________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com