
“ÓŃõ»Æ»¹Ō·“Ó¦µÄ½Ē¶Čæ“£¬ŌŚÖʱøĻĀĮŠČżÖÖĘųĢåŹ±£¬ŃĪĖįĘšŹ²Ć“×÷ÓĆ£ŗ£ØĢīŠņŗÅ£©
£Ø1£©ÖʱøH2 Zn+2HCl=ZnCl2+H2”ü ŃĪĖįĘš______×÷ÓĆ
£Ø2£©ÖʱøCl2 MnO2+4HCl =MnCl2 +Cl2”ü+2H2O ŃĪĖįĘš______×÷ÓĆ
£Ø3£©ÖʱøCO2 CaCO3+2HCl=CaCl2+CO2”ü+H2O ŃĪĖįĘš______×÷ÓĆ
A£®Ńõ»Æ¼Į B£®»¹Ō¼Į
C£®¼ČŹĒŃõ»Æ¼ĮÓÖŹĒ»¹Ō¼Į D£®¼Č²»ŹĒŃõ»Æ¼ĮŅ²²»ŹĒ»¹Ō¼Į
 ŠĀĖ¼Ī¬¼ŁĘŚ×÷Ņµŗ®¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
ŠĀĖ¼Ī¬¼ŁĘŚ×÷Ņµŗ®¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğ°²»ÕŹ”øßŅ»ÉĻĘŚÖŠ²āŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
Ļņŗ¬8.0g NaOHµÄČÜŅŗÖŠĶØČėŅ»¶ØĮæH2Sŗ󣬽«µĆµ½µÄČÜŅŗŠ”ŠÄÕōøÉ£¬³ĘµĆĪŽĖ®Īļ7.9g£¬ŌņøĆĪŽĖ®ĪļÖŠŅ»¶Øŗ¬ÓŠµÄĪļÖŹŹĒ£Ø £©
A£®Na2S B£®NaHS C£®Na2SŗĶNaHS D£®NaOHŗĶNaHS
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016½ģĮÉÄžŹ”øßČżÉĻѧʌʌ֊²āŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠÓŠ¹ŲµÄ¼ĘĖć·ÖĪö²»ÕżČ·µÄŹĒ£Ø £©
A£®ŌŚ·“Ó¦3Cu£«8HNO3(Ļ”)=3Cu(NO3)2£«2NO”ü£«4H2OÖŠ£¬µ±ÓŠ1 molĶ±»Ńõ»ÆŹ±£¬±»»¹ŌµÄĻõĖįĪŖ2/3mol
B£®½«a molĮņ»ÆŃĒĢś·ÅČėŗ¬3a mol H2SO4µÄÅØĮņĖįÖŠ£¬³ä·Ö·“Ó¦ŗó£¬Ńõ»Æ”¢»¹Ō²śĪļ·Ö±šŹĒFe3£«”¢SŗĶSO2£¬Ōņ·Å³öµÄĘųĢåÉŁÓŚ1.5a mol
C£®ŹŅĪĀŹ±£¬ŌŚČŻ»żĪŖa mLµÄŹŌ¹ÜÖŠ³äĀśNO2ĘųĢå£¬Č»ŗóµ¹ÖĆŌŚĖ®ÖŠµ½¹ÜÄŚĖ®Ćę²»ŌŁÉĻÉżŹ±ĪŖÖ¹£»ŌŁĶØČėb mL O2£¬Ōņ¹ÜÄŚŅŗĆęÓÖ¼ĢŠųÉĻÉż£¬²āµĆŹŌ¹ÜÄŚ×īŗóŹ£ÓąĘųĢåĪŖc mL£¬ĒŅøĆĘųĢå²»ÄÜÖ§³ÖČ¼ÉÕ”£Ōņa”¢bµÄ¹ŲĻµĪŖa£½4b£«3c
D£®Ä³ČÜŅŗ100 mL£¬ĘäÖŠŗ¬ĮņĖį0.03 mol£¬ĻõĖį0.04 mol£¬ČōŌŚøĆČÜŅŗÖŠĶ¶Čė1.92 gĶ·ŪĪ¢ČČ£¬·“Ó¦ŗó·Å³öŅ»Ńõ»ÆµŖĘųĢåŌ¼ĪŖ0.015 mol
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğøŹĖąŹ”ø߶žÉĻĘŚÖŠ£ØĪÄ£©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
±ź×¼×“æöĻĀ£¬22.4LCO2µÄĪļÖŹµÄĮæĪŖ
A£®0.20mol B£®0.50mol C£®1.0mol D£®2.0mol
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğŗ£ÄĻŹ”ø߶žÉĻ12ŌĀ¶Īæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
ĄūÓĆÓŅĶ¼×°ÖĆ²ā¶ØÖŠŗĶČȵďµŃé²½ÖčČēĻĀ£ŗ
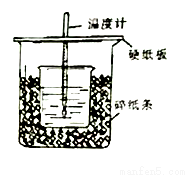
¢ŁĮæČ”50mL 0.25mol•L-1ĮņĖįµ¹ČėŠ”ÉÕ±ÖŠ£¬²āĮæĪĀ¶Č£»
¢ŚĮæČ”50mL 0.55mol•L-1NaOHČÜŅŗ£¬²āĮæĪĀ¶Č£»
¢Ū½«NaOHČÜŅŗµ¹ČėŠ”ÉÕ±ÖŠ£¬»ģŗĻ¾łŌČŗ󣬲āĮæ»ģŗĻŅŗĪĀ¶Č”£Ēė»Ų“š£ŗ
£Ø1£©“ÓŹµŃé×°ÖĆÉĻæ“£¬Ķ¼ÖŠÉŠČ±ÉŁµÄŅ»ÖÖ²£Į§ÓĆĘ·ŹĒ_____________£»
£Ø2£©NaOHČÜŅŗÉŌ¹żĮæµÄŌŅņ_____________£»
£Ø3£©¼ÓČėNaOHČÜŅŗµÄÕżČ·²Ł×÷ŹĒ______£ØĢī×ÖÄø£©£®
A£®ŃŲ²£Į§°ō»ŗĀż¼ÓČė B£®Ņ»“ĪŃøĖŁ¼ÓČė C£®·ÖČż“Ī¼ÓČė
£Ø4£©Ź¹ĮņĖįÓėNaOHČÜŅŗ»ģŗĻ¾łŌȵÄÕżČ·²Ł×÷ŹĒ_______£»
£Ø5£©ÉčČÜŅŗµÄĆÜ¶Č¾łĪŖ1g/cm3£¬ÖŠŗĶŗóČÜŅŗµÄ±ČČČČŻc=4.18J/£Øg•”ę£©£¬Ēėøł¾ŻŹµŃ鏿¾ŻŠ“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ _____________£»
ĪĀ¶Č ŹµŃé“ĪŹż | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Č t2/”ę | ĪĀ¶Č²īĘ½¾łÖµ£Øt2-t1£©/”ę | ||
H2SO4 | NaOH | Ę½¾łÖµ | |||
1 | 25.0 | 25.2 | 25.1 | 28.5 | |
2 | 24.9 | 25.1 | 25.0 | 28.3 | |
3 | 25.5 | 26.5 | 26 | 31.8 | |
4 | 25.6 | 25.4 | 25.5 | 29.0 | |
£Ø6£©ÉĻŹöŹµŃ鏿ֵ½į¹ūÓė57.3kJ/molÓŠĘ«²ī£¬²śÉśĘ«²īµÄŌŅņæÉÄÜŹĒ£ØĢī×ÖÄø£© _______
a£®ŹµŃé×°ÖƱ£ĪĀ”¢øōČČŠ§¹ū²ī
b£®·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĮņĖįµÄŠ”ÉÕ±ÖŠ
c£®ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č
£Ø7£©______£ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©ÓĆBa(OH)2“śĢęĒāŃõ»ÆÄĘČÜŅŗŗĶĻ”ĮņĖį·“Ó¦£»Čō½«ŗ¬0.5molH2SO4µÄ ÅØĮņĖįÓėŗ¬1molNaOHµÄČÜŅŗ»ģŗĻ£¬·Å³öµÄČČĮæ________£ØĢī”°Š”ÓŚ”±”¢”°µČÓŚ”±»ņ”°“óÓŚ”±£©57.3kJ”£ŌŅņŹĒ_______________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğŗ£ÄĻŹ”øßŅ»ÉĻ12ŌĀ¶Īæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
¼ų±š½ŗĢåŗĶČÜŅŗ×ī¼ņµ„µÄ·½·ØŹĒ
A£®¶”“ļ¶ūŠ§Ó¦ B£®ÕōĮó C£®¹żĀĖ D£®ŻĶČ”
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğŗÓ±±Ź”ø߶žÉĻ12ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
XŗĶYĮ½ÖÖŌŖĖŲµÄŗĖµēŗÉŹżÖ®ŗĶĪŖ22£¬XµÄŌ×ÓŗĖĶāµē×ÓŹż±ČYµÄÉŁ6øö”£ĻĀĮŠĖµ·ØÖŠ²»ÕżČ·µÄŹĒ ( )
A£®XµÄµ„ÖŹ¹ĢĢ¬Ź±ĪŖ·Ö×Ó¾§Ģå
B£®YµÄµ„ÖŹĪŖŌ×Ó¾§Ģå
C£®XÓėYŠĪ³ÉµÄ»ÆŗĻĪļ¹ĢĢ¬Ź±ĪŖ·Ö×Ó¾§Ģå
D£®XÓėĢ¼ŠĪ³ÉµÄ»ÆŗĻĪļĪŖ·Ö×Ó¾§Ģå
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğ½ĖÕŹ”ø߶žÉĻµŚ¶ž“ĪÖŹ¼ģ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠĪļÖŹ²»ŹōÓŚĢģČ»øß·Ö×Ó»ÆŗĻĪļµÄŹĒ
A£®µķ·Ū B£®²ĻĖæ C£®ĘĻĢŃĢĒ D£®ĻĖĪ¬ĖŲ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğÕć½Ź”ø߶žÉĻĘŚÖŠ²āŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠ±ä»Æ¹ż³ĢÖŠ£¬Ć»ÓŠ·¢Éś»Æѧ±ä»ÆµÄŹĒ
A£®ÓĆÕōĮó·Øµ»Æŗ£Ė® B£®“Óŗ£“ųÖŠĢįČ”µā
C£®ŹÆÄ«×Ŗ»ÆĪŖ½šøÕŹÆ D£®°±ĘųČܽāŌŚĖ®ÖŠ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com