
ij�л���A����ʽΪCxHyOz,15 g A��ȫȼ������22 g CO2��9 g H2O��
(1)���л�������ʽ��____________��
(2)��A��һ����ɫ����ǿ�Ҵ̼�����ζ�����壬���л�ԭ�ԣ�����ṹ��ʽ��__________��
(3)��A��Na2CO3���������ų����ʹ��ܷ���������Ӧ����A�Ľṹ��ʽΪ______________��
(4)��A���ӷ���ˮ����ζ��Һ�壬�ܷ���ˮ�ⷴӦ������ṹ��ʽΪ____________��
(5)������ӽṹ�к���6��̼ԭ�ӣ����ж�Ԫ����ȩ�����ʣ�����ṹ��ʽΪ____________��
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���������ͬһ��Һ��һ���ܴ����������(����)
A.������Ba2+����Һ��:Cl-��K+��S ��C
��C
B.������H+����Һ��:Mg2+��Na+��C ��S
��S
C.������OH-����Һ��:K+��N ��S
��S ��Cu2+
��Cu2+
D.������Na+����Һ��:H+��K+��S ��N
��N
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA��ʾ�����ӵ�������ֵ������������ȷ����
A��1 L 0.2mol��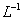 CH3COONa��Һ�к���0.2NA��CH3COO��
CH3COONa��Һ�к���0.2NA��CH3COO��
B����״���£�11.2LCl2����ˮ��ת�Ƶĵ�����ΪNA
C�����³�ѹ�£�23g NO2��N2O4�Ļ�����庬�е�ԭ����Ϊ1.5NA
D��100 mL 18.4mol��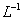 Ũ����������ͭ���ȷ�Ӧ������SO2�ķ�����Ϊ0.92NA
Ũ����������ͭ���ȷ�Ӧ������SO2�ķ�����Ϊ0.92NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��Ӧԭ���ڿ��к�ũҵ�������й㷺Ӧ�á�
 (1)ij��ѧ��ȤС����й�ҵ�ϳɰ���ģ���о�����Ӧ�ķ���ʽΪN2(g)+3H2(g)
(1)ij��ѧ��ȤС����й�ҵ�ϳɰ���ģ���о�����Ӧ�ķ���ʽΪN2(g)+3H2(g) 2NH3(g)
2NH3(g)  ����lL�ܱ������м���0.1 mol N2��0.3mol H2��ʵ��١��ڡ�����c(N2)��ʱ��
����lL�ܱ������м���0.1 mol N2��0.3mol H2��ʵ��١��ڡ�����c(N2)��ʱ�� �ı仯����ͼ��ʾ��
�ı仯����ͼ��ʾ��
ʵ��ڴӳ�ʼ��ƽ��Ĺ����У��÷�Ӧ��ƽ����Ӧ����v(NH3)=__________________����ʵ�����ȣ�ʵ��ں�ʵ������ı��ʵ�������ֱ�Ϊ����ѡ���е�__________��__________(����ĸ���)��
a������ѹǿ b����Сѹǿ C�������¶�d�������¶� e��ʹ�ô���
(2)��֪NO2��N2O4�����ת���� ��
��
��T��ʱ����0.40 mol NO2��������ݻ�Ϊ2L���ܱ������У��ﵽƽ����������c(N2O4)=0.05 mol��L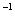 ����÷�Ӧ��ƽ�ⳣ��K=_______________��
����÷�Ӧ��ƽ�ⳣ��K=_______________��
����֪N2O4�ڽϸ��¶��������ȶ����ڣ���ת��ΪNO2���������¶ȣ�������Ӧ��ƽ�ⳣ��K��_____________(���������С�����䡱)��
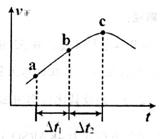 ��������ܱ�������ͨ��һ������NO2��ijʱ���������Ӧ������ʱ�ʵı仯��ͼ��ʾ������˵����ȷ����__________(����ĸ���)��
��������ܱ�������ͨ��һ������NO2��ijʱ���������Ӧ������ʱ�ʵı仯��ͼ��ʾ������˵����ȷ����__________(����ĸ���)��
A����Ӧ��c��ﵽƽ��״̬
B����Ӧ��Ũ�ȣ�a��С��b��
C��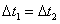 ʱ��NO2��ת���ʣ�a��b��С�� b��c��
ʱ��NO2��ת���ʣ�a��b��С�� b��c��
(3)25��ʱ����amol��L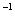 �İ�ˮ��b mol��Lһ1����������ϣ���Ӧ����Һǡ�������ԣ���a___________b(�>������<����=��)����a��b��ʾNH3
�İ�ˮ��b mol��Lһ1����������ϣ���Ӧ����Һǡ�������ԣ���a___________b(�>������<����=��)����a��b��ʾNH3 H2O�ĵ���ƽ�ⳣ��
H2O�ĵ���ƽ�ⳣ�� =________________��
=________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���д������
(���� )
A��ͨ�����Ǻ͵��۶����Ի�ԭ��
B���ô�����ľ���һ���������ܷⱣ�棬ʱ��Խ��Խ�㴼
C����ά�ء����ǡ������Ǻ�֬����һ�������¶��ɷ���ˮ�ⷴӦ
D����ά�ط������������ǵ�Ԫ��ɵģ����Ա��ֳ�һЩ��Ԫ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪ij���������Է�������С��200����������������ԼΪ0.5�����������̼�ĸ������Ϊ
(���� )
A��5�� B��6��
C��7�� D��8��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ɼ����һ�����Ϳ���ҩ�������ʽΪC47H51NO14�����������µ�A���B�����ɵ�һ������
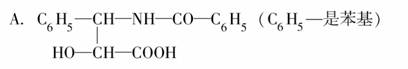
B��R��OH(R��һ����C��H��O�Ļ���)
(1)A�����������ˮ�⣬�䷴Ӧ����ʽ��
__________________________________________________________________
________________________________________________________________��
(2)Aˮ�����õİ��������Ȼ�����ʵ�ˮ������Ϊ��������(��ϣ����ĸ)________λ��
(3)д��ROH�ķ���ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������зֱ������ᣬ�������ͬ�������(����)
�ٴ���ʯ��������ʯ����ˮ�����ܱ��ǣ��ݵ���
A���٢� B���ܢ�
C���٢ڢ� D���٢ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NH4HCO3���Թ��м���,ʹ�ų�����������ͨ��ʢ�������������Ƶĸ���ܡ�����Ũ�����ϴ��ƿ,���õ���������(����)
A.NH3������ B.O2���� ��C.H2O������ D.CO2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com