
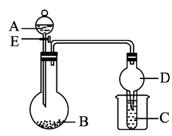
ͼ1-5-14
(1)��AΪŨ���BΪ�������ڽ���Ԫ�ص�Ƭ״���ʣ����ڳ���������ˮ��Ӧ��CΪƷ����Һ��ʵ���й۲쵽��Һ��ɫ����B��_____________(д��ѧʽ)��B��ŨH2SO4��Ӧ�Ļ�ѧ����ʽΪ_____________����Ӧ�����ձ��м����ˮ���ֿɹ۲쵽�Թ�C�е�����Ϊ__________________________��
(2)��BΪNa2CO3��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ����ǣ�����AӦ���е�������____________________��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е�������___________________��
(3)��B����ʯ�ң�ʵ���й۲쵽C��Һ���γɳ�����Ȼ������ܽ⣬����Һǡ�ó���ʱ���ر�E��Ȼ�����ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��_____________ (д����)��C��_____________ (д��ѧʽ)�������ǵĻ��Һ���÷�Ӧ�����ӷ���ʽΪ_____________������D�ڴ�ʵ���е�������__________________________��
����:�����ۺϿ���������Mg��Al��H2SO4��Na2CO3��NH3��H2O���л��ﱽ�ӡ������ǵ�֪ʶ��Ӧ�ã������൱��֪ʶ��Ⱥ�˼ά���������е�(1)�ʣ�B��������ԭ���dz��������ۻ�����(2)�ʣ������ˮ�����ӵ��ܽ������(3)�ʣ�D�����ݻ��ɷ�����(�����ڵ���©��)��
��:(1)Mg Mg+2H2SO4(Ũ)====MgSO4+SO2��+2H2O C��Һ���
(2)���Ա�̼��ǿ ����Һ�����
(3)Ũ��ˮ AgNO3
CH2OH(CHOH)4CHO+2[Ag(NH3)2��++2OH-![]() CH2OH(CHOH)4COO-+
CH2OH(CHOH)4COO-+![]() +2Ag��+3NH3+H2O ��ֹ����
+2Ag��+3NH3+H2O ��ֹ����


 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����£���0.10mol?L-1KOH��Һ�ζ�10.00mL 0.10mol?L-1H2C2O4��Һ���õĵζ�������ͼ��ʾ�������Һ������ɿ��ɻ��ǰ����Һ�����֮�ͣ�����ش��������⣺
�����£���0.10mol?L-1KOH��Һ�ζ�10.00mL 0.10mol?L-1H2C2O4��Һ���õĵζ�������ͼ��ʾ�������Һ������ɿ��ɻ��ǰ����Һ�����֮�ͣ�����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
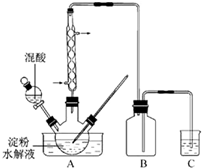 ��2011?���գ�������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ��
��2011?���գ�������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��)ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ���ṩ���Լ�:��ϸ�ĸ�Ƭ��ĩ (��Ƭ�е������ɷֲ������ᷴӦ)��![]() ���ᡢ5%NaOH��Һ������
���ᡢ5%NaOH��Һ������![]() ��Һ������
��Һ������![]() ��Һ������ˮ��
��Һ������ˮ��
ʵ�����:
��.�������װ�õ������ԡ�
��.��A��C���ұ���0.25g��Ƭ��ĩ�������3mL![]() ���ᣬ�������ӡ���B��E�о����뱥��
���ᣬ�������ӡ���B��E�о����뱥��![]() ��Һ,��ͼ��ʾ�����������ܶ�����
��Һ,��ͼ��ʾ�����������ܶ�����
��.��A��C��б��ʹҺ��������ϣ�ʵ���������ȴ����������ܶ��������B���ռ���������Ϊ41.90mL��E���ռ������������Ϊ39.20mL(�������������������Ϊ��״���µ��������
�ش���������:
(1)���м��ͼ1װ�������Եķ�����
��
��2��A�з�����Ӧ�����ӷ���ʽΪ ��
D�м����Լ�Ϊ ��D�������� ��
��3��ʵ��ǰ��������Һ����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ���еIJ�����
��
��4��ͼ2ʵ�����ø�Ƭ�е�̼��Ƶ���������Ϊ ;ͼ1ʵ���ͼ 2ʵ�����ø�Ƭ�е�̼��ƺ���ƫ�ߣ����������ʽ��ʾƫ�ߵ�ԭ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡΫ��������һУ������ѧ��ģ���⻯ѧ�Ծ� ���ͣ������
(14��)ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ���ṩ���Լ�:��ϸ�ĸ�Ƭ��ĩ (��Ƭ�е������ɷֲ������ᷴӦ)�� ���ᡢ5%NaOH��Һ������
���ᡢ5%NaOH��Һ������ ��Һ������
��Һ������ ��Һ������ˮ��
��Һ������ˮ��

ʵ�����:
��.�������װ�õ������ԡ�
��.��A��C���ұ���0.25g��Ƭ��ĩ�������3mL ���ᣬ�������ӡ���B��E�о����뱥��
���ᣬ�������ӡ���B��E�о����뱥�� ��Һ,��ͼ��ʾ�����������ܶ�����
��Һ,��ͼ��ʾ�����������ܶ�����
��.��A��C��б��ʹҺ��������ϣ�ʵ���������ȴ����������ܶ��������B���ռ���������Ϊ41.90mL��E���ռ������������Ϊ39.20mL(�������������������Ϊ��״���µ��������
�ش���������:
(1)���м��ͼ1װ�������Եķ�����
��
��2��A�з�����Ӧ�����ӷ���ʽΪ ��
D�м����Լ�Ϊ ��D�������� ��
��3��ʵ��ǰ��������Һ����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ���еIJ�����
��
��4��ͼ2ʵ�����ø�Ƭ�е�̼��Ƶ���������Ϊ ;ͼ1ʵ���ͼ 2ʵ�����ø�Ƭ�е�̼��ƺ���ƫ�ߣ����������ʽ��ʾƫ�ߵ�ԭ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com