
 ̼���⡢����������ȷǽ���Ԫ�����������������ϢϢ��أ��ش��������⣺
̼���⡢����������ȷǽ���Ԫ�����������������ϢϢ��أ��ش��������⣺| 1 |
| 8 |
| 1 |
| 2 |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A��Xһ���ǻ��ý��� |
| B��Y����������������������� |
| C��Z�ĵ�����˫ԭ�ӷ��� |
| D���⻯����ȶ��ԣ�Y��X |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ñ���ȡ��ˮ�е�Br2ʱ���л���ӷ�Һ©�����¶˷ų� |
| B��������Һ���Ƿ���SO42-�����ε���Ba��NO3��2��Һ��ϡ���� |
| C��������Һʱ������ˮ��������ƿ�̶ȣ�Ӧ�ý�ͷ�ιܽ�������Һ���� |
| D������AlCl3��Һʱ����AlCl3�ܽ��ڽ�Ũ�������У�����ˮϡ�͵�����Ũ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH4�ȱ������ֱ���ԭ |
| B��H2O�Ȳ����������ֲ��ǻ�ԭ�� |
| C���ڷ�Ӧ�����£�ÿ����1mol H2O��Ȼ����67.2L H2 |
| D������ƽ���ʹ�ø���Ч�Ĵ�������ʹH2�IJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼ��ͼ��
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼ��ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| T/K | T1 | T2 | T3 |
| K | 1.00��107 | 2.54��105 | 1.88��103 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
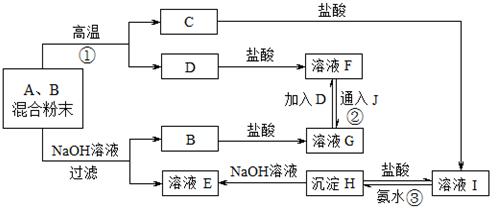
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com