

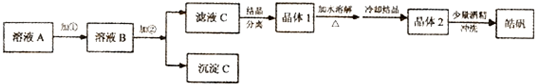
·ÖĪö Öʱø2Zn£ØOH£©2•ZnCO3Į÷³ĢĪŖ£ŗŠæ±ŗÉ°£ØÖ÷ŅŖ³É·ÖĪŖZnO£¬ŗ¬ÉŁĮæCu2+”¢Mn2+µČĄė×Ó£©ÖŠ¼ÓČėĮņĖįļ§”¢°±Ė®”¢Ė«ŃõĖ®£¬Ė«ŃõĖ®½«ĆĢĄė×ÓŃõ»Æ³É¶žŃõ»ÆĆĢ£¬¹żĀĖŗóŌŚĀĖŅŗÖŠ¼ÓĮņ»Æļ§£¬ŌŁ¹żĀĖ£¬³żČ„ĶĄė×Ó£¬Õō°±³żČ„¶ąÓąµÄ°±Ęų£¬¼ÓČėĢ¼ĖįĒāļ§µĆµ½2Zn£ØOH£©2•ZnCO3ŗĶ¶žŃõ»ÆĢ¼ĘųĢ壬¹żĀĖµĆ2Zn£ØOH£©2•ZnCO3£¬ĀĖŅŗĪŖĮņĖįļ§ČÜŅŗ£¬
£Ø1£©£ØNH4£©2SO4ÓėNH3•H2OµÄ»ģŗĻČÜŅŗÖŠ“ęŌŚµēŗÉŹŲŗć£ŗc £ØNH4+£©+c£ØH+£©=2c£ØSO42-£©+c£ØOH-£©£¬ŌŁ½įŗĻc£ØNH4+£©=2c£ØSO42-£©ÅŠ¶ĻČÜŅŗĖį¼īŠŌ£»
£Ø2£©øł¾ŻÓ°Ļģ·“Ó¦ĖŁĀŹµÄŅņĖŲÅŠ¶Ļ”°½žČ””±Ź±ĪŖĮĖĢįøߊæµÄ½ž³öĀŹ£¬æɲÉČ”µÄ“ėŹ©£»
£Ø3£©”°½žČ””±Ź±¼ÓČėµÄNH3•H2O¹żĮ棬ČÜŅŗ³Ź¼īŠŌ£¬Ė«ŃõĖ®½«ĆĢĄė×ÓŃõ»Æ³ÉMnO2£¬øł¾ŻµēŗÉŹŲŗćŗĶŌŖĖŲŹŲŗćŹéŠ“Ąė×Ó·½³ĢŹ½£»
£Ø4£©øł¾Ż·“Ó¦ZnS+Cu2+=CuS+Zn2+£¬æÉÖŖK=$\frac{Ksp£ØZnS£©}{Ksp£ØCuS£©}$=1.2”Į1012£¬½įŗĻĢāÖŠŠÅĻ¢æÉÅŠ¶ĻZnS³żŌÓ£¬ŹĒ·ńæÉŠŠ£»
£Ø5£©”°³ĮŠæ”±µÄ¹ż³ĢĪŖČÜŅŗÖŠµÄŠæĄė×ÓÓėĢ¼ĖįĒāļ§ČÜŅŗ·“Ӧɜ³É2Zn£ØOH£©2•ZnCO3£¬¾Ż“ĖŹéŠ“Ąė×Ó·½³ĢŹ½£»
£Ø6£©¹żĀĖ²Ł×÷ŠčŅŖÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠÉÕ±”¢²£Į§°ō”¢Ā©¶·µČ£¬³ĮµķĪüø½ĮņĖįøłĄė×Ó£¬æÉÓĆ¼ģŃéĮņĖįøłĄė×ӵķ½·Ø¼ģŃéŹĒ·ńĻ“µÓøɾ»£®
½ā“š ½ā£ŗ£Ø1£©NH4£©2SO4ÓėNH3•H2OµÄ»ģŗĻČÜŅŗÖŠ“ęŌŚµēŗÉŹŲŗć£ŗc £ØNH4+£©+c£ØH+£©=2c£ØSO42-£©+c£ØOH-£©£¬µ±c£ØNH4+£©=2c£ØSO42-£©Ź±£¬c£ØH+£©=c£ØOH-£©£¬¼“ČÜŅŗĻŌÖŠŠŌ£¬
¹Ź“š°øĪŖ£ŗÖŠ£»
£Ø2£©øł¾ŻÓ°Ļģ·“Ó¦ĖŁĀŹµÄŅņĖŲÅŠ¶Ļ”°½žČ””±Ź±ĪŖĮĖĢįøߊæµÄ½ž³öĀŹ£¬æɲÉČ”µÄ“ėŹ©ĪŖ½Į°č”¢ŹŹµ±¼ÓČČ£¬Ņ²æÉŃÓ³¤½žČ”Ź±¼ä”¢¶ą“Ī½žČ”£¬
¹Ź“š°øĪŖ£ŗ½Į°č”¢ŹŹµ±¼ÓČČ”¢ŃÓ³¤½žČ”Ź±¼ä”¢¶ą“Ī½žČ”µČ£»
£Ø3£©”°½žČ””±Ź±¼ÓČėµÄNH3•H2O¹żĮ棬ČÜŅŗ³Ź¼īŠŌ£¬Ė«ŃõĖ®½«ĆĢĄė×ÓŃõ»Æ³ÉMnO2£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖMn2++H2O2+2NH3•H2O=MnO2”ż+2NH4++2H2O£¬
¹Ź“š°øĪŖ£ŗMn2++H2O2+2NH3•H2O=MnO2”ż+2NH4++2H2O£»
£Ø4£©øł¾Ż·“Ó¦ZnS+Cu2+=CuS+Zn2+£¬æÉÖŖK=$\frac{Ksp£ØZnS£©}{Ksp£ØCuS£©}$=1.2”Į1012£¾£¾1”Į105£¬K£¾105»Æѧ·“Ó¦ĶźČ«£¬ĖłŅŌŃ”ŌńZnS½ųŠŠ³żŌÓŹĒæÉŠŠµÄ£¬
¹Ź“š°øĪŖ£ŗæÉŠŠ£¬ZnS+Cu2+?CuS+Zn2+£¬K=$\frac{Ksp£ØZnS£©}{Ksp£ØCuS£©}$=1.2”Į1012£¾£¾1”Į105£»
£Ø5£©”°³ĮŠæ”±µÄ¹ż³ĢĪŖČÜŅŗÖŠµÄŠæĄė×ÓÓėĢ¼ĖįĒāļ§ČÜŅŗ·“Ӧɜ³É2Zn£ØOH£©2•ZnCO3£¬Ąė×Ó·½³ĢŹ½ĪŖ3Zn2++6HCO3-=2Zn£ØOH£©2•ZnCO3”ż+5CO2”ü+H2O£¬
¹Ź“š°øĪŖ£ŗ3Zn2++6HCO3-=2Zn£ØOH£©2•ZnCO3”ż+5CO2”ü+H2O£»
£Ø6£©¹żĀĖ²Ł×÷ŠčŅŖÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠÉÕ±”¢²£Į§°ō”¢Ā©¶·µČ£¬³ĮµķĪüø½ĮņĖįøłĄė×Ó£¬æÉÓĆ¼ģŃéĮņĖįøłĄė×ӵķ½·Ø¼ģŃéŹĒ·ńĻ“µÓøɾ»£¬·½·ØŹĒČ”×īŗóŅ»“ĪĻ“µÓŅŗÉŁĮæÓŚŹŌ¹ÜÖŠ£¬¼ÓČėŃĪĖįĖį»ÆµÄĀČ»Æ±µČÜŅŗ£¬ĪŽ°×É«³ĮµķÉś³É£¬
¹Ź“š°øĪŖ£ŗÉÕ±”¢²£Į§°ō”¢Ā©¶·£»Č”×īŗóŅ»“ĪĻ“µÓŅŗÉŁĮæÓŚŹŌ¹ÜÖŠ£¬¼ÓČėŃĪĖįĖį»ÆµÄĀČ»Æ±µČÜŅŗ£¬ĪŽ°×É«³ĮµķÉś³É£®
µćĘĄ ±¾Ģāæ¼²éĮĖĪļÖŹÖʱø·½°øµÄÉč¼Ę”¢ĪļÖŹ·ÖĄėÓėĢį“æ·½·ØµÄ×ŪŗĻÓ¦ÓĆ£¬ĪŖøßĘµæ¼µćŗĶ³£¼ūĢāŠĶ£¬ĢāÄæÄŃ¶Č½Ļ“ó£¬Ć÷Č·ÖʱøĮ÷³ĢĪŖ½ā“š¹Ų¼ü£¬×¢ŅāÕĘĪÕ³£¼ūĪļÖŹ·ÖĄėÓėĢį“æµÄ²Ł×÷·½·Ø£¬ŹŌĢāÖŖŹ¶µć½Ļ¶ą”¢×ŪŗĻŠŌ½ĻĒ棬³ä·Öæ¼²éĮĖѧɜµÄ·ÖĪö”¢Ąķ½āÄÜĮ¦¼°»ÆѧŹµŃéÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| æŖŹ¼³Įµķ | ³ĮµķĶźČ« | |
| Fe£ØOH£©3 | 2.7 | 3.7 |
| Fe£ØOH£©2 | 7.6 | 9.6 |
| Zn£ØOH£©2 | 5.7 | 8.0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĘųĢå¼×Ņ»¶ØŹĒ“æ¾»Īļ | |
| B£® | ³Įµķ¼×ŹĒ¹čĖįŗĶ¹čĖįøʵĻģŗĻĪļ | |
| C£® | Na+”¢AlO2-ŗĶSiO32-Ņ»¶Ø“ęŌŚÓŚČÜŅŗXÖŠ | |
| D£® | CO32-ŗĶSO42-Ņ»¶Ø²»“ęŌŚÓŚČÜŅŗXÖŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1£¬1-¶žĀČŅŅĻ© | B£® | ±ūĻ© | C£® | 1-¶”Ļ© | D£® | 2-ĪģĻ© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆ“æ¼īČÜŅŗČܽā±½·Ó£ŗCO32-+C6H5OH”śC6H5O-+HCO3- | |
| B£® | ÓĆĒæ¼īČÜŅŗĪüŹÕ¹¤ŅµÖĘČ”ĻõĖįĪ²ĘųNO+NO2+2OH-=2NO3-+H2O | |
| C£® | ÓƶžŃõ»ÆĆĢŗĶÅØŃĪĖį·“Ó¦ÖĘĀČĘų£ŗMnO2+4HCl£ØÅØ£© $\frac{\underline{\;\;”÷\;\;}}{\;}$Mn2++2Cl-+Cl2”ü+2H2O | |
| D£® | ĻņAlCl3ČÜŅŗÖŠµĪ¼Ó¹żĮæµÄ°±Ė®£ŗAl3++4NH3•H2O=AlO2-+2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŚøĆ·“Ó¦Ģõ¼žĻĀ£¬MgµÄ»¹ŌŠŌĒæÓŚCµÄ»¹ŌŠŌ | |
| B£® | Mg”¢MgOÖŠĆ¾ŌŖĖŲĪ¢Į£µÄ°ė¾¶£ŗr£ØMg2+£©£¾r£ØMg£© | |
| C£® | ŌŖĖŲCµÄµ„ÖŹÖ»“ęŌŚ½šøÕŹÆŗĶŹÆÄ«Į½ÖÖĶ¬ĖŲŅģŠĪĢå | |
| D£® | øĆ·“Ó¦ÖŠ»ÆѧÄÜČ«²æ×Ŗ»ÆĪŖČČÄÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com