

���� ��1������������Һ���ѡ����ʹ������ƿ������m=CVM������Ҫ���ʵ�������
��2���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��3������һ�����ʵ���Ũ�ȵ���Һ�IJ����У����㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ�����ͼ�Т١������ʵ������жϲ�������������
��� �⣺��1������0.2mol•L-1��Na2CO3��Һ480mL��Ӧѡ��500mL����ƿ����Ҫ���ʵ�����m=0.2mol•L-1��0.5L��286g/mol=28.6g��
�ʴ�Ϊ��28.6g��
��2����Na2CO3•10H2O����ʧȥ�˲��ֽᾧˮ���������ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ�
���á���������ij��������������壨ʹ�����룩��ʵ�ʳ�������ƫС�����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
��̼���ƾ��岻�������л����Ȼ��ƣ��������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
�ܳ���̼���ƾ���ʱ�����������⣬���³�ȡ�����ʵ�����ƫ����ҺŨ��ƫ�ߣ�
������ƿδ������ʹ�ã������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣻
���ԣ�����������ҺŨ��ƫ�ߵ��Т٢ܣ�ƫ�͵��Тڢۣ���Ӱ����Тݣ�
�ʴ�Ϊ���٢ܣ��ڢۣ��ݣ�
�� 3���ڵڢ۲��͵ڢܲ�֮�仹��Ҫϴ���ձ��Ͳ��������ڢݶ���ʱ������Ҫ������ƿ�еİ�Һ����ƽ�������ܹ���������
��ѡB��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ����жϣ���Ŀ�ѶȲ���Ҫ��ѧ��������������һ�����ʵ���Ũ�ȵ���Һ�IJ��輰��ȷ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��

| A�� | �����¶Ⱥͻ���λ�ò��䣬�ڼ����ټ���1 mol A��2 mol B���ﵽ�µ�ƽ�����C��Ũ��������C��Ũ�ȵ�2�� | |
| B�� | ���ֻ���λ�ò��䣬�����¶ȣ��ﵽ�µ�ƽ��ס�����B��������������� | |
| C�� | �����¶Ⱥ����е�ѹǿ���䣬t2ʱ�ֱ���ס����м���������ĺ����ס����з�Ӧ���ʱ仯����ֱ���ͼ2��ͼ3��ʾ��t1ǰ�ķ�Ӧ���ʱ仯��ʡ�ԣ� | |
| D�� | �����¶Ȳ��䣬�ƶ�����P��ʹ�ҵ��ݻ��ͼ���ȣ��ﵽ�µ�ƽ�������C����������Ǽ���C �����������2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��Ba2+��S2-��SO42- | B�� | Na+��Cu2+��SO42-��Cl- | ||
| C�� | Br-��Ba2+��Cl-��K+ | D�� | Ca2+��K+��CO32-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ij����С��������ij��������Ʒ���Ƿ����������ƣ���Ʒ������£�ȡ�������������Һ������Һ�еμ���������KMnO4��Һ���۲���Һ�Ƿ���ɫ�������Һ��ɫ��ȥ��˵������Ʒ�к����������ƣ�
ij����С��������ij��������Ʒ���Ƿ����������ƣ���Ʒ������£�ȡ�������������Һ������Һ�еμ���������KMnO4��Һ���۲���Һ�Ƿ���ɫ�������Һ��ɫ��ȥ��˵������Ʒ�к����������ƣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
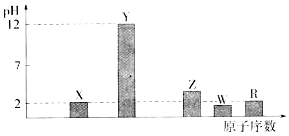
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������λ��Ԫȡ����ֻ��һ�� | B�� | ���ļ�λ��Ԫȡ����ֻ��һ�� | ||
| C�� | ���Ķ�λ��Ԫȡ����ֻ��һ�� | D�� | ������λ��Ԫȡ�����ж��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com