
ʯī�ڲ�����������ҪӦ�á�ij����ʯī�к�SiO2(7.8%)��Al2O3(5.1%)��Fe2O3(3.1%)��MgO(0.5%)�����ʡ���Ƶ��ᴿ���ۺ����ù������£�
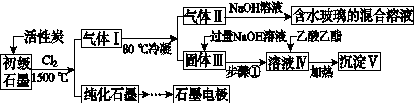
(ע��SiCl4�ķе�Ϊ57.6 �棬�����Ȼ���ķе������150 ��)
(1)��Ӧ����ͨ��Cl2ǰ����ͨһ��ʱ��N2����ҪĿ����____________________��
(2)���·�Ӧ��ʯī�����������ʾ�ת��Ϊ��Ӧ���Ȼ��������е�̼��������ҪΪ________�����������ij��õ�ˮ�����Ļ�ѧ��Ӧ����ʽΪ____________________________________________��
(3)�����Ϊ�����衢________��������Һ���е���������________��
(4)����Һ�����ɳ��������ܷ�Ӧ�����ӷ���ʽΪ______________________________________________��100 kg����ʯī�����ܻ�â�������Ϊ______kg��
(5)ʯī��������Ȼˮ����ͭ���ĵ绯ѧ�����������ͼ����ʾ��ͼ��������Ӧ��ע��

32��(1)�ų�װ���еĿ��� ��
(2)CO�� SiCl4��6NaOH===Na2SiO3��4NaCl��3H2O ��
(3)���ˡ�AlO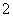 ��Cl�� ��
��Cl�� ��
(4)AlO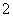 ��CH3COOCH2CH3��2H2O
��CH3COOCH2CH3��2H2O CH3COO����CH3CH2OH��Al(OH)3����7.8 ��
CH3COO����CH3CH2OH��Al(OH)3����7.8 ��
(5)
[����] (1)ͨ��N2��Ŀ����Ϊ���ų�װ���еĿ���(�ر�������)����ֹ�ڸ���ʱʯī��������Ӧ��(2)���·�Ӧ��SiO2��Al2O3��Fe2O3��MgO�ֱ�ת��ΪSiCl4��AlCl3��FeCl3��MgCl2����Ϊʯī�ǹ����ģ��ʸ��������£�C��SiO2��Fe2O3��Ӧ���ɵ���CO������SiCl4�ķе�Ϊ57.6 �棬����80 �棬�������ΪSiCl4����NaOH��Һ��ˮ������Na2SiO3��NaCl: SiCl4��6NaOH===Na2SiO3��4NaCl��3H2O��(3)AlCl3��FeCl3��MgCl2�ķе������150 �棬��80 ���±�Ϊ����� AlCl3��FeCl3��MgCl2����NaOH��Ӧ������NaAlO2��Fe(OH)3��Mg(OH)2��NaCl��ͨ�����˽�����Fe(OH)3��Mg(OH)2�˳����õ�����Һ����Ҫ��NaAlO2��NaCl��(4)NaAlO2����ˮ�����Һ�Լ��ԣ�NaAlO2��2H2O Al(OH)3��NaOH������������������ˮ�⣺CH3COOCH2CH3��NaOH
Al(OH)3��NaOH������������������ˮ�⣺CH3COOCH2CH3��NaOH CH3COONa��CH3CH2OH����ʹNaAlO2����ˮ������Al(OH)3��������Һ��ת��Ϊ�������ķ�ӦΪNaAlO2��2H2O��CH3COOCH2CH3
CH3COONa��CH3CH2OH����ʹNaAlO2����ˮ������Al(OH)3��������Һ��ת��Ϊ�������ķ�ӦΪNaAlO2��2H2O��CH3COOCH2CH3 Al(OH)3����CH3COONa��CH3CH2OH������Alԭ���غ㣬��֪100 kg����ʯī�ɵ�m[Al(OH)3]��
Al(OH)3����CH3COONa��CH3CH2OH������Alԭ���غ㣬��֪100 kg����ʯī�ɵ�m[Al(OH)3]��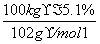 ��2��78 g��mol��1��7.8 kg��
��2��78 g��mol��1��7.8 kg��
(5)ˮ����ͭ���ĵ绯ѧ���������˵�Ᵽ�������÷�����ʯī��������ͭ��������������ӵ���������������������ʯī��ͭ��ֱ���������γ�ԭ��أ���ͭ�����������������Բ��ɲ��á�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ����(����)
A��Cl2ͨ��NaOH��Һ��
Cl2��OH��===Cl����ClO����H2O
B��NaHCO3��Һ�м���ϡ���
CO ��2H��===CO2����H2O
��2H��===CO2����H2O
C��AlCl3��Һ�м������ϡ��ˮ��
Al3����4NH3��H2O===AlO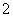 ��4NH
��4NH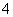 ��2H2O
��2H2O
D��Cu����ϡHNO3��
3Cu��8H����2NO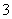 ===3Cu2����2NO����4H2O
===3Cu2����2NO����4H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ͻ��ǽ��캽��ĸ����������ϡ�
(1) ��ĸ�������������Ͻ����졣
�� ��Ԫ�������ڱ��е�λ��Ϊ ����ҵ������ԭ������������ȡ���á���ȡ������ͨ�������Ϊ ��
�� Al-Mg�Ͻ�ǰ��NaOH��Һ����Al2O3Ĥ���仯ѧ����ʽΪ ��
���ӹ�����ʹ�õı�����Ϊ (�ѧʽ)��
(2) ��ĸ����Ϊ�Ͻ�֡�
�� �����ں�ˮ�з����ĵ绯ѧ��ʴ��ҪΪ ��
�� ��ĸ�øֿ��ɵ�����ұ�����ɣ���������������Ϊ���躬������������Ϊ ��
(3) ��ĸ��������Ҫ��ͭ�Ͻ����졣
�� 80.0gCu-Al�Ͻ�������ȫ�ܽ���������ˮ�����˵ð�ɫ����39.0g����Ͻ���Cu����������Ϊ ��
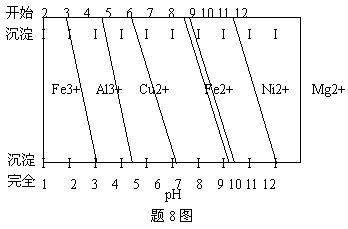
�� Ϊ����ijͭ�Ͻ�ijɷ֣����Ὣ����ȫ�ܽ����NaOH��Һ��pH����pH��3.4ʱ��ʼ���ֳ������ֱ���pHΪ7.0��8.0ʱ���˳����������8ͼ��Ϣ�ƶϸúϽ��г�ͭ��һ������ ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ���ɻ�ͭ��(��Ҫ�ɷ�CuFeS2)ұ��ͭ����Ҫ�������£�
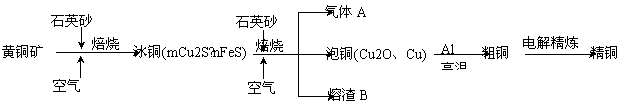

(1)����A�еĴ�����Ⱦ���ѡ�������Լ��е�_______���ա�
a��ŨH2SO4 b��ϡHNO3 c��NaOH��Һ d����ˮ
(2)��ϡH2SO4��������B��ȡ����������Һ���μ�KSCN��Һ��ʺ�ɫ��˵����Һ�д��� (�� ���ӷ���)��������Һ�л��� ��Fe2���ķ����� (ע���Լ�������)��
��Fe2���ķ����� (ע���Լ�������)��
(3)����ͭұ����ͭ�Ļ�ѧ��Ӧ����ʽΪ ��
(4)��CuSO4��ҺΪ�������Һ���д�ͭ(��Al��Zn��Ag��Pt��Au������)�ĵ�⾫��������˵����ȷ���� ��
a������ȫ��ת��Ϊ��ѧ�� b����ͭ�ӵ�Դ����������������Ӧ
c����Һ��Cu2���������ƶ� ����������d������������ɻ���Ag��Pt��Au�Ƚ���
(5)���÷�Ӧ2Cu+O2+2H2SO4=2CuSO4+2H2O���Ʊ�CuSO4�������÷�Ӧ���Ϊԭ��أ��������缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�������Ӧ�ú���ɵ�˵����ȷ����(����)
A��P2O5�����ڸ���Cl2��NH3
B������ȼ������Ҫ�ɷ��Ǽ����ˮ
C��CCl4�����ڼ�����ˮ�͵�ˮ
D��Si��SiO2������������ά
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ǧ���仯������������ء������豸��X���߷������ϵȡ��ش��������⣺
(1)Ǧ��̼��ͬ��Ԫ�أ���̼��4�����Ӳ㡣Ǧ��Ԫ�����ڱ���λ��Ϊ��________���ڡ���________�壻PbO2�����Ա�CO2������________(�ǿ��������)��
(2)PbO2��Ũ���Ṳ�����ɻ���ɫ���壬��Ӧ�Ļ�ѧ����ʽΪ_______________________��
(3)PbO2����PbO�����������Һ��Ӧ�Ƶã���Ӧ�����ӷ���ʽΪ___________________��PbO2Ҳ����ͨ��ʯīΪ�缫��Pb(NO3)2��Cu(NO3)2�Ļ����ҺΪ���Һ�����ȡ�����������ĵ缫��ӦʽΪ____________________�������Ϲ۲쵽��������____________________�������Һ�в�����Cu(NO3)2�����������ĵ缫��ӦʽΪ______________________________������������Ҫȱ����____________________��
(4)PbO2�ڼ��ȹ��̷����ֽ��ʧ����������ͼ��ʾ����֪ʧ�������ϵ�a��Ϊ��Ʒʧ��4.0%(��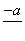 ��100%)�IJ������塣��a�������ɱ�ʾΪPbOx��mPbO2��nPbO����ʽ����xֵ��m��nֵ_______________________________________
��100%)�IJ������塣��a�������ɱ�ʾΪPbOx��mPbO2��nPbO����ʽ����xֵ��m��nֵ_______________________________________
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I����)�л��յ⣬��ʵ��������£�
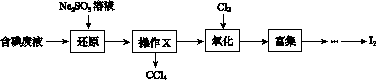
(1)���Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ__________________���ò�����I2��ԭΪI����Ŀ����______________________��
(2)����X������Ϊ________��
(3)����ʱ����������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����40 �����ҷ�Ӧ(ʵ��װ����ͼ��ʾ)��
ʵ������ڽϵ��¶��½��е�ԭ����______________����ƿ��ʢ�ŵ���ҺΪ________��

(4)��֪��5SO ��2IO
��2IO ��2H��===I2��5SO
��2H��===I2��5SO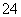 ��H2O
��H2O
ij�����ˮ(pHԼΪ8)��һ������I2�����ܴ���I����IO �е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO
�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO ��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϳ����ú����ˮ����Na2S2O3��5H2O��ʵ���ҿ�������װ��(��ȥ���ּг�����)ģ���������̡�
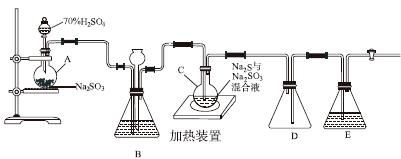
��ƿC�з�����Ӧ���£�
Na2S(aq)��H2O(l)��SO2(g)===Na2SO3(aq)��H2S(aq)��(��)
2H2S(aq)��SO2(g)===3S(s)��2H2O(l)��(��)
S(s)��Na2SO3(aq)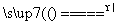 Na2S2O3(aq)��(��)
Na2S2O3(aq)��(��)
(1)������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һ������________________��������װ�����������á�װ��D��������__________��װ��E��Ϊ________��Һ��
(2)Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2S��Na2SO3ǡ����ȫ��Ӧ������ƿC��Na2S��Na2SO3���ʵ���֮��Ϊ________��
(3)װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ��________��
a������ˮ b������Na2SO3��Һ
c������NaHSO3��Һ d������NaHCO3��Һ
ʵ���У�ΪʹSO2����������ƿC�����õIJ�����__________________________����֪��Ӧ(��)��Խ���������ƿC�з�Ӧ�ﵽ�յ��������__________________����Ӧ���ڿ��þƾ����ʵ�������ƿA��ʵ�����þƾ��Ƽ���ʱ����ʹ��ʯ��������������________��
a���ձ� b��������
c���Թ� d����ƿ
(4)��Ӧ��ֹ����ƿC�е���Һ������Ũ������ȴ�ᾧ��������Na2S2O3��5H2O�����п��ܺ���Na2SO3��Na2SO4�����ʡ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ�________________________________________��
��֪Na2S2O3��5H2O�����ֽ⣺S2O ��2H��===S����SO2����H2O
��2H��===S����SO2����H2O
��ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ԭ�ϣ��ýӴ�����������Ĺ����У��Է�����Դ�Ĵ�������ȷ���� (����)��
A�������ᷨβ���������Ƚ�β���ð�ˮ���գ�Ȼ������Ũ���ᴦ��
B����β����������ȣ���ת��������Ͷ�ʽ��٣������з�����Խϸ�
C�����Դ��������ų��������л���������ɫ�����Լ����ؽ��������
D������¯�Կ������á����ȡ���¯���������Ȳ������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com