
����Ŀ������(CH3OCH3)��һ��������Դ��
��֪��CO(g)+2H2(g)CH3OH(g) H1=-99kJ/mol
��2CH3OH(g)CH3OCH3(g)+H2O(g) H2=-24kJ/mol
��CO(g)+H2O(g)CO2(g)+H2(g) H3=-41kJ/mol
�ش��������⣺
(1)д��CO��H2��Ӧ����CO2��CH3OCH3(g)���Ȼ�ѧ����ʽ��________________��
(2)���д�ʩ����߷�Ӧ����CO��ƽ��ת���ʵ���________(����ĸ)��
A.����ѹǿ B.�����¶� C.����H2Ũ�� D.�Ӹ�Ч����
(3)�ں��º���������ֻ������Ӧ�ۣ�������������÷�Ӧ�ﵽƽ�����________(����ĸ)��
A.����ѹǿ���ֲ��� B.�����ܶȱ��ֲ���
C.![]() ���ֲ��� D.Ũ���̱��ֲ���
���ֲ��� D.Ũ���̱��ֲ���
(4)��һ���¶�(T��)�£�������ܱ�������Ͷ��һ����CH3OH���壬ֻ������Ӧ�ڡ�����������CH3OCH3�����ʵ�������[��(CH3OCH3)]�뷴Ӧʱ��(t)���й����������ʾ��
t/min | 0 | 15 | 30 | 45 | 80 | 100 |
[��(CH3OCH3)] | 0 | 0.05 | 0.08 | 0.09 | 0.10 | 0.10 |
��30minʱ��CH3OH��ת������(CH3OH)________�������¶��£�������Ӧ��ƽ�ⳣ��K=________���÷�����ʾ����
�ڷ�Ӧ����v=v��-v��������v��=k����2(CH3OH)��v��=k����(CH3OCH3)��(H2O)��k����k���ֱ�Ϊ�����淴Ӧ���ʳ�����ֻ���¶��йأ���Ϊ���ʵ���������15minʱ![]() ________(�������2λС��)
________(�������2λС��)
(5)���ܱ������з�����Ӧ�ۣ�ƽ�ⳣ��ΪK��pK=-lg2K��pK�¶ȵĹ�ϵ��ͼ��ʾ��ͼ������________������a������b�����ܷ�ӳƽ�ⳣ���仯���ơ�

(6)��CO2�������ı���KHCO3��Һ�У������CO2�Ʊ�CH3OCH3��ԭ����ͼ��ʾ��������HCO3-���ɣ��õ�ĵ缫��ӦʽΪ________��

���𰸡�3CO(g)+3H2(g)CO2(g)+CH3OCH3(g) H=-263kJ/mol AB CD 16 ![]() 5.06 a 14CO2+12e-+9H2O=CH3COH3+12HCO3-
5.06 a 14CO2+12e-+9H2O=CH3COH3+12HCO3-
��������
(1)���ݸ�˹����2��+��+���ɵ�����
(2)������������ԭ���жϡ�
(3)��Ӧ��������ļ���������������ȣ���Ӧ��ʼ���գ�������ѹǿ�����ʵ������ܶȲ��䣬������Ϊƽ��ı�־����Ӧƽ��ʱ��n(CO)��n(CO2)�������ٸı䣬Ũ�Ȳ��ٸı䣻
(4)���ݱ������ݣ���Ӧ��80minʱ�ﵽƽ��״̬����(CH3OCH3)Ϊ0.10��
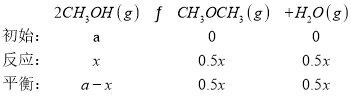
(5)��Ӧ�����ʱ�С���㣬Ϊ���ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ�ⳣ����С��
(6)��������õ�����CO2����CH3OCH3ʱ����HCO3-���ɡ�
(1)��3CO(g)+3H2(g)CO2(g)+CH3OCH3(g)�����ݸ�˹����2��+��+���ɵ�����3CO(g)+3H2(g)CO2(g)+CH3OCH3(g) H=-263kJ/mol��
(2)��߷�Ӧ����CO��ƽ��ת���ʣ���ƽ�������ƶ���������������ԭ������ѹǿ�ͽ����¶ȿ�ʹƽ�������ƶ��������������������ΪAB��
(3)��Ӧ��������ļ���������������ȣ���Ӧ��ʼ���գ�������ѹǿ�����ʵ������ܶȲ��䣬������Ϊƽ��ı�־����Ӧƽ��ʱ��n(CO)��n(CO2)�������ٸı䣬Ũ�Ȳ��ٸı䣬��ΪCD��
(4)���ݱ������ݣ���Ӧ��80minʱ�ﵽƽ��״̬����(CH3OCH3)Ϊ0.10��
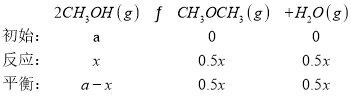
��30minʱ����������ʽ��![]() =0.08�����x=0.16a��CH3OH��ת������(CH3OH)=
=0.08�����x=0.16a��CH3OH��ת������(CH3OH)=![]() ��100%=16%��ͬ����ƽ��ʱx=0.2��K=
��100%=16%��ͬ����ƽ��ʱx=0.2��K=![]() =
=![]() ��
��
��15minʱ����������ʽ��![]() =0.05�����x=0.1a������(CH3OCH3)=��(H2O)=0.05a����2(CH3OH)=(0.9a)2��ƽ��ʱ��v��=k����2(CH3OH)=v��=k����(CH3OCH3)��(H2O)��
=0.05�����x=0.1a������(CH3OCH3)=��(H2O)=0.05a����2(CH3OH)=(0.9a)2��ƽ��ʱ��v��=k����2(CH3OH)=v��=k����(CH3OCH3)��(H2O)��![]() =K��
=K�� =
=![]() =5.06��
=5.06��
(5)��Ӧ�����ʱ�С���㣬Ϊ���ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ�ⳣ����С��pK=-lg2K����pK����a�ܷ�ӳƽ�ⳣ���仯���ƣ�
(6)��CO2�������ı���KHCO3��Һ�У���������õ��ӣ���CO2����CH3OCH3ʱ����HCO3-���ɣ��缫��ӦʽΪ14CO2+12e-+9H2O=CH3COH3+12HCO3-��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ����( )
A.60g�������辧���к���2NA��������B.1molD2O�к���10NA������
C.12g���ʯ�к���NA��̼̼��D.1molʯī�����к���2NA��̼̼��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������ŷɴ��Ļ��ȼ�ϳ�Һ̬˫��ˮ�⣬������һ��Һ̬���⻯�����֪�û���������Ԫ�ص���������Ϊ12.5������Է�������Ϊ32���ṹ�������ָ÷��ӽṹ��ֻ�е�����
��1���õ��⻯����ĵ���ʽΪ_________��
��2����������Һ̬˫��ˮ��Ӧ�ܲ����������ֲ���Ⱦ���������ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ_______________________��
��3��NH3�����е�Nԭ����һ�Թ¶Ե��� �ܷ�����Ӧ��NH3+HCl=NH4Cl����д���������⻯����ͨ����������ʱ��������Ӧ�ķ���ʽ��
�ܷ�����Ӧ��NH3+HCl=NH4Cl����д���������⻯����ͨ����������ʱ��������Ӧ�ķ���ʽ��
_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������H��һ���л��������м��塣ʵ�����ɷ��㻯����A�Ʊ�H��һ�ֺϳ�·�����£�
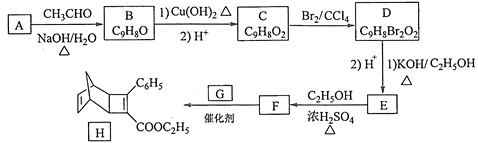
��֪����RCHO+CH3CHO ![]() RCH=CHCHO+H2O����
RCH=CHCHO+H2O����![]() ��
��
�ش��������⣺
(1)C�Ľṹ��ʽΪ_______________________��
(2)F�й����ŵ�����Ϊ___________________��
(3)B������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽΪ________________��
(4)��ת������ͼ�ɼ�����D��E���������У����е�һ����Ӧ������Ϊ__________��
(5)������G�Ķ��ȴ�����__________��ͬ���칹�塣
(6)���㻯����X��D��ͬ���칹�壬X�ܷ���������Ӧ����˴Ź���������ʾ��3�ֻ�ѧ�������⣬�����֮��Ϊ6��1��1������������X�Ľṹ���ж��֣���д��2�ַ���Ҫ���X�Ľṹ��ʽ________��___________��
(7)д���ü�ȩ����ȩΪԭ���Ʊ�������CH2=CHCOOCH3�ĺϳ�·�ߣ������Լ���ѡ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��ϵͳ������������
���CH3CH(C2H5)CH(CH3)2��������________________________��
(2)д�����и����л���Ľṹ��ʽ��
��2,3����4�һ�����_________________��
��֧��ֻ��һ���һ�����Է���������С������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() Ϊ�����ӵ�������ֵ������˵����ȷ����
Ϊ�����ӵ�������ֵ������˵����ȷ����
A. 0.1 mol ��![]() ������
������![]() ������
������
B. pH=1��H3PO4��Һ�У�����![]() ��
��![]()
C. 2.24L����״��������O2����ȫȼ�գ��õ�![]() ��CO2����
��CO2����
D. �ܱ�������1 mol PCl3��1 mol Cl2��Ӧ�Ʊ� PCl5��g��������![]() ��P-Cl��
��P-Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ�� X��Y��Z��W ԭ����������������X �ǵؿ��к�������Ԫ����Y ԭ�ӵ������ֻ��һ��������Z λ��Ԫ�����ڱ���A����W ��X����ͬһ���塣����˵����ȷ����
A. ԭ�Ӱ뾶��r(W) > r(Z) > r(Y)
B. ��X��Y ��ɵĻ������о��������ۼ�
C. Y ������������ˮ����ļ��Ա�Z����
D. X �ļ���̬�⻯������ȶ��Ա�W��ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ���л������У��е���ͬ���칹�壬�е�û��ͬ���칹�壬����һ��������ͬ���칹��ķ�Ӧ��
A.�����ϩ(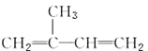 )������ʵ�����Br2�����ӳɷ�Ӧ
)������ʵ�����Br2�����ӳɷ�Ӧ
B.2���ȶ�����NaOH�Ҵ���Һ���ȷ�����ȥHCl���ӵķ�Ӧ
C.�ױ���һ�������·���������Ӧ����һ�����ױ��ķ�Ӧ
D.������Na2CO3��Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1Lij��Һ�к��е��������±���
���� |
|
|
|
|
���ʵ���Ũ��
| 1 | 1 |
| 1 |
�ö��Ե缫������Һ������·����![]() ͨ��ʱ�����Ե��ʱ��Һ����ı仯���缫������ܴ��ڵ��ܽ���������˵����ȷ����
ͨ��ʱ�����Ե��ʱ��Һ����ı仯���缫������ܴ��ڵ��ܽ���������˵����ȷ����
A.��������Һ��pH=0B.a=3
C.��������![]() D.���������Ľ�����ͭ����
D.���������Ľ�����ͭ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com