
ijŠ£»ÆѧѧĻ°Š”×éÄāŃéÖ¤NOÄܱ»°±Ęų»¹Ō²¢²ā¶ØĘä×Ŗ»ÆĀŹ£¬Éč¼ĘČēĻĀŹµŃé£ŗ
²Īæ¼ÖŖŹ¶£ŗNOĘųĢåÓėFeSO4ČÜŅŗ·“Ӧɜ³ÉæÉČÜŠŌĮņĖįŃĒĻõ»łŗĻĢś£Ø+2¼Ū£©£ŗNO+FeSO4=Fe(NO)SO4

ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ

¢Å æÉÓĆÉĻĶ¼ÖŠ×°ÖĆ £ØĢī±ąŗÅ£¬¶ąŃ”£©½ųŠŠ°±ĘųµÄŹµŃéŹŅÖʱø”£
D×°ÖĆæÉÓĆÓŚĻĀĮŠ £ØĢī±ąŗÅ£¬¶ąŃ”£©ĘųĢåµÄÖʱø”£
a CO2 b O2 c SO2 d C2H4 e C2H2 f H2
¢Ę ×°ÖĆ¢Ū”¢¢ÜČōÓĆĶ¬Ņ»×°ÖĆ£¬ŌņøĆ×°ÖĆÖŠĖłŹ¢×°µÄŅ©Ę·ŹĒ £ØĢī±ąŗÅ£©
A£®ĀČ»ÆøĘ B£®ÅØĮņĖį C£®ĪåŃõ»Æ¶žĮ× D£®¼īŹÆ»Ņ
¢Ē ×°ÖĆ¢ŽÖŠ£¬“Ö²£Į§¹ÜXµÄ×÷ÓĆ £¬×°ÖĆ¢ßµÄ×÷ÓĆ
¢Č ×°ÖĆ¢ŽµÄ¹ćæŚĘæÖŠ£¬³żÉś³ÉFe(NO)SO4Ķā£¬»¹ÓŠ°×É«³ĮµķÉś³É£¬Š“³öÉś³ÉøĆ³ĮµķµÄĄė×Ó·½³ĢŹ½
¢É µ±½ųČė×°ÖĆ¢ŻµÄNOĪŖ2.688L£Ø±ź×¼×“æö£¬ĻĀĶ¬£©£¬°±Ęų¹żĮ棬×īŗóŹÕ¼Æµ½2.016LN2£¬ŌņNOµÄ×Ŗ»ÆĀŹĪŖ ”£
£Ø9·Ö£©£Ø1£©ABC£Ø2·Ö£© af£Ø1·Ö£© (2) D£Ø1·Ö£©
£Ø3£©·ĄÖ¹µ¹Īü£Ø1·Ö£©£»³żČ„Ī“·“Ó¦µÄ°±Ęų²¢øÉŌļN2£Ø1·Ö£©
£Ø4£©Fe2£«+2NH3+2H2O=Fe(OH)2”ż+2NH4£« £Ø1·Ö£© £Ø5£©90£„ £Ø2·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©ŹµŃéŹŅÖʱø°±ĘųæÉŅŌÓĆŹģŹÆ»ŅÓėĀČ»Æļ§¼ÓČČ£¬¼“×°ÖĆAÕżČ·£»Ņ²æÉŅŌ½«°±Ė®µĪČėµ½ÉśŹÆ»Ņ»ņ¼īŹÆ»ŅÖŠ£¬ŌņBÕżČ·£¬»ņÕßÖ±½Ó¼ÓČČÅØ°±Ė®£¬CæÉŅŌ£¬“š°øŃ”ABC”£D×°ÖĆŹĒ¼ņŅ×ĘōĘÕ·¢ÉśĘ÷£¬æÉŅŌŌĄ“ÖʱøĒāĘųŗĶCO2£¬“š°øŃ”af”£
£Ø2£©°±ĘųŹĒ¼īŠŌĘųĢ壬Ōņ²»ÄÜÓĆĖįŠŌøÉŌļ¼Į£¬Ņ²²»ÄÜÓĆĀČ»ÆøĘøÉŌļ°±Ęų£¬Ōņ“š°øŃ”D”£
£Ø3£©°±Ęų¼«Ņ×ČÜÓŚĖ®£¬ĖłŅŌ×°ÖĆ¢ŽÖŠ£¬“Ö²£Į§¹ÜXµÄ×÷ÓĆ·ĄÖ¹µ¹Īü£»ŅŖŹÕ¼ÆµŖĘų£¬ŌņŠčŅŖ³żČ„µŖĘųÖŠµÄ°±Ęų£¬ĖłŅŌÅØĮņĖįµÄ×÷ÓĆŹĒ³żČ„Ī“·“Ó¦µÄ°±Ęų²¢øÉŌļN2”£
£Ø4£©°±ĘųČÜÓŚĖ®Éś³É°±Ė®£¬ČÜŅŗĻŌ¼īŠŌ£¬ŗĶŃĒĢśĄė×Ó·“Ӧɜ³ÉĒāŃõ»ÆŃĒĢś°×É«³Įµķ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒFe2£«+2NH3+2H2O£½Fe(OH)2”ż+2NH4£«”£
£Ø5£©NOµÄĪļÖŹµÄĮæŹĒ2.688L”Ā22.4L/mol£½0.12mol£¬µŖĘųµÄĪļÖŹµÄĮæŹĒ2.016L”Ā22.4L/mol£½0.09mol£¬Ōņøł¾Ż·“Ó¦µÄ·½³ĢŹ½4NH3£«6NO£½5N2£«6H2OæÉÖŖĻūŗÄNOŹĒ0.108mol£¬ĖłŅŌNOµÄ×Ŗ»ÆĀŹŹĒ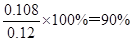 ”£
ӣ
æ¼µć£ŗ漲鰱ĘųÖʱø”¢øÉŌļ¼ĮµÄŃ”Ōń”¢»ł±¾ŹµŃé²Ł×÷ŅŌ¼°·½³ĢŹ½µÄŹéŠ“ŗĶ×Ŗ»ÆĀŹµÄ¼ĘĖć
µćĘĄ£ŗøĆĢāŹĒøßæ¼ÖŠµÄ³£¼ūæ¼µćŗĶĢāŠĶ£¬ŹōÓŚÖŠµČÄѶȏŌĢāµÄ漲飬ŹŌĢā×ŪŗĻŠŌĒ棬²ąÖŲ¶ŌѧɜÄÜĮ¦µÄÅąŃųŗĶ½āĢā·½·ØµÄÖøµ¼ÓėѵĮ·£¬Ö¼ŌŚæ¼²éѧɜĮé»īŌĖÓĆ»ł“”ÖŖŹ¶½ā¾öŹµ¼ŹĪŹĢāµÄÄÜĮ¦£¬ÓŠĄūÓŚÅąŃųѧɜµÄĀß¼ĶĘĄķÄÜĮ¦ŗĶ¹ę·¶ŃĻ½÷µÄŹµŃéÉč¼ĘÄÜĮ¦£¬ĢįøßѧɜµÄѧæĘĖŲŃų”£øĆĢāµÄ¹Ų¼üŹĒĆ÷Č·ŹµŃéŌĄķ£¬²¢ÄܽįŗĻ×°ÖĆĮé»īŌĖÓĆ¼“æÉ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŠ£»ÆѧѧĻ°Š”×éÄāŃéÖ¤NOÄܱ»°±Ęų»¹Ō²¢²ā¶ØĘä×Ŗ»ÆĀŹ£¬Éč¼ĘČēĻĀŹµŃé£ŗ
²Īæ¼ÖŖŹ¶£ŗNOĘųĢåÓėFeSO4ČÜŅŗ·“Ӧɜ³ÉæÉČÜŠŌĮņĖįŃĒĻõ»łŗĻĢś(¢ņ)£ŗNO+FeSO4=Fe(NO)SO4
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ÅŹµŃéŹŅÓĆĀČ»Æļ§ÓėĒāŃõ»ÆøĘ¹ĢĢåÖʱø°±Ęų£¬Ó¦Ń”ÓƵÄ×°ÖĆŹĒ
øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½
ŹµŃéŹŅĶس£ÓĆ ·ØŹÕ¼Æ°±Ęų£¬¼ģŃéøĆĘųĢåŹĒ·ńŹÕ¼ÆĀś·½·Ø
¢Ę×°ÖĆ¢Ū”¢¢ÜČōÓĆĶ¬Ņ»×°ÖĆ£¬ŌņøĆ×°ÖĆÖŠĖłŹ¢×°µÄŅ©Ę·ŹĒ
A£®Na2O2 B£®ÅØĮņĖį C£®P2O5 D£®¼īŹÆ»Ņ
¢Ē×°ÖĆ¢ŽÖŠ£¬“Ö²£Į§¹ÜXµÄ×÷ÓĆ £¬×°ÖĆ¢ßµÄ×÷ÓĆ
¢Č×°ÖĆ¢ŽµÄ¹ćæŚĘæÖŠ£¬³żÉś³ÉFe(NO)SO4Ķā£¬»¹ÓŠ°×É«³ĮµķÉś³É£¬Š“³öÉś³ÉøĆ³ĮµķµÄĄė×Ó·½³ĢŹ½
¢ÉČō²Ī¼Ó·“Ó¦µÄNOĪŖ2.688L£Ø±ź×¼×“æö£¬ĻĀĶ¬£©£¬°±Ęų¹żĮ棬×īŗóŹÕ¼Æµ½2.016LN2£¬Ōņ×°ÖĆ¢ŻÖŠNOµÄ×Ŗ»ÆĀŹĪŖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģÕć½Ź”ŗ¼ÖŻ¶žÖŠøßČż6ŌĀČČÉśæ¼£ØĄķ×Ū£©»ÆѧŹŌĢā ĢāŠĶ£ŗŹµŃéĢā
ijŠ£»ÆѧѧĻ°Š”×éÄāŃéÖ¤NOÄܱ»°±Ęų»¹Ō²¢²ā¶ØĘä×Ŗ»ÆĀŹ£¬Éč¼ĘČēĻĀŹµŃé£ŗ
²Īæ¼ÖŖŹ¶£ŗNOĘųĢå ÓėFeSO4ČÜŅŗ·“Ӧɜ³ÉæÉČÜŠŌĮņĖįŃĒĻõ»łŗĻĢś(¢ņ)£ŗNO+FeSO4=Fe(NO)SO4
ÓėFeSO4ČÜŅŗ·“Ӧɜ³ÉæÉČÜŠŌĮņĖįŃĒĻõ»łŗĻĢś(¢ņ)£ŗNO+FeSO4=Fe(NO)SO4
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ÅŹµŃéŹŅÓĆĀČ»Æļ§ÓėĒāŃõ»ÆøĘ¹ĢĢåÖʱø°±Ęų£¬Ó¦Ń”ÓƵÄ×°ÖĆŹĒ
øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½
ŹµŃéŹŅĶس£ÓĆ ·ØŹÕ¼Æ°±Ęų£¬¼ģŃéøĆĘųĢåŹĒ·ńŹÕ¼ÆĀś·½·Ø
¢Ę×°ÖĆ¢Ū”¢¢ÜČōÓĆĶ¬Ņ»×°ÖĆ£¬ŌņøĆ×°ÖĆÖŠĖłŹ¢×°µÄŅ©Ę·ŹĒ
| A£®Na2O2 | B£®ÅØĮņĖį | C£®P2O5 | D£®¼īŹÆ»Ņ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģŗÓ±±Ź”ŗāĖ®ÖŠŃ§øßČżµŚČż“ĪÄ£Äāæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
ijŠ£»ÆѧѧĻ°Š”×éÄāŃéÖ¤NOÄܱ»°±Ęų»¹Ō²¢²ā¶ØĘä×Ŗ»ÆĀŹ£¬Éč¼ĘČēĻĀŹµŃé£ŗ
²Īæ¼ÖŖŹ¶£ŗNOĘųĢåÓėFeSO4ČÜŅŗ·“Ӧɜ³ÉæÉČÜŠŌĮņĖįŃĒĻõ»łŗĻĢś£Ø+2¼Ū£©£ŗNO+FeSO4=Fe(NO)SO4
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Å æÉÓĆÉĻĶ¼ÖŠ×°ÖĆ £ØĢī±ąŗÅ£¬¶ąŃ”£©½ųŠŠ°±ĘųµÄŹµŃéŹŅÖʱø”£
D×°ÖĆæÉÓĆÓŚĻĀĮŠ £ØĢī±ąŗÅ£¬¶ąŃ”£©ĘųĢåµÄÖʱø”£
a CO2 b O2 c SO2 d C2H4 e C2H2 f H2
¢Ę ×°ÖĆ¢Ū”¢¢ÜČōÓĆĶ¬Ņ»×°ÖĆ£¬ŌņøĆ×°ÖĆÖŠĖłŹ¢×°µÄŅ©Ę·ŹĒ £ØĢī±ąŗÅ£©
| A£®ĀČ»ÆøĘ | B£®ÅØĮņĖį | C£®ĪåŃõ»Æ¶žĮ× | D£®¼īŹÆ»Ņ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğÕć½Ź”øßČż6ŌĀČČÉśæ¼£ØĄķ×Ū£©»ÆѧŹŌĢā ĢāŠĶ£ŗŹµŃéĢā
ijŠ£»ÆѧѧĻ°Š”×éÄāŃéÖ¤NOÄܱ»°±Ęų»¹Ō²¢²ā¶ØĘä×Ŗ»ÆĀŹ£¬Éč¼ĘČēĻĀŹµŃé£ŗ
²Īæ¼ÖŖŹ¶£ŗNOĘųĢåÓėFeSO4ČÜŅŗ·“Ӧɜ³ÉæÉČÜŠŌĮņĖįŃĒĻõ»łŗĻĢś(¢ņ)£ŗNO+FeSO4=Fe(NO)SO4

ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ

¢ÅŹµŃéŹŅÓĆĀČ»Æļ§ÓėĒāŃõ»ÆøĘ¹ĢĢåÖʱø°±Ęų£¬Ó¦Ń”ÓƵÄ×°ÖĆŹĒ
øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½
ŹµŃéŹŅĶس£ÓĆ ·ØŹÕ¼Æ°±Ęų£¬¼ģŃéøĆĘųĢåŹĒ·ńŹÕ¼ÆĀś·½·Ø
¢Ę×°ÖĆ¢Ū”¢¢ÜČōÓĆĶ¬Ņ»×°ÖĆ£¬ŌņøĆ×°ÖĆÖŠĖłŹ¢×°µÄŅ©Ę·ŹĒ
A£®Na2O2 B£®ÅØĮņĖį C£®P2O5 D£®¼īŹÆ»Ņ
¢Ē×°ÖĆ¢ŽÖŠ£¬“Ö²£Į§¹ÜXµÄ×÷ÓĆ £¬×°ÖĆ¢ßµÄ×÷ÓĆ
¢Č×°ÖĆ¢ŽµÄ¹ćæŚĘæÖŠ£¬³żÉś³ÉFe(NO)SO4Ķā£¬»¹ÓŠ°×É«³ĮµķÉś³É£¬Š“³öÉś³ÉøĆ³ĮµķµÄĄė×Ó·½³ĢŹ½
¢ÉČō²Ī¼Ó·“Ó¦µÄNOĪŖ2.688L£Ø±ź×¼×“æö£¬ĻĀĶ¬£©£¬°±Ęų¹żĮ棬×īŗóŹÕ¼Æµ½2.016LN2£¬Ōņ×°ÖĆ¢ŻÖŠNOµÄ×Ŗ»ÆĀŹĪŖ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com