
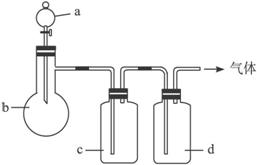
Нј15-8
ЖшМе | a | b | c | D |
C2H4 | ТТґј | ЕЁH2SO4 | NaOHИЬТє | ЕЁH2SO4 |
Cl2 | ЕЁСОЛб | MnO2 | NaOHИЬТє | ЕЁH2SO4 |
NH3 | ±ҐєНNH4ClИЬТє | ПыКЇ»Т | H2O | №ММеNaOH |
NO | ПЎHNO3 | НРј | H2O | P2O5 |
ЈЁ1Ј©ЙПКц·Ѕ·ЁЦРїЙТФµГµЅёЙФпЎўґїѕ»µДЖшМеКЗ___________ЎЈ
ЈЁ2Ј©ЦёіцІ»ДЬУГЙПКц·Ѕ·ЁЦЖИЎµДЖшМеЈ¬ІўЛµГчАнУЙЈЁїЙТФІ»МоВъЈ©ЎЈ
ўЩЖшМе___________Ј¬АнУЙКЗ__________________________________________ЎЈ
ўЪЖшМе___________Ј¬АнУЙКЗ__________________________________________ЎЈ
ўЫЖшМе___________Ј¬АнУЙКЗ__________________________________________ЎЈ
ўЬЖшМе___________Ј¬АнУЙКЗ__________________________________________ЎЈ
ЅвОцЈєЦЖ±ёC2H4РиїШЦЖОВ¶ИФЪ170 ЎжЈ¬Ч°ЦГЦРОЮОВ¶ИјЖЈ¬ФтОЮ·ЁїШЦЖОВ¶ИЈ»јУИИЕЁСОЛбУлMnO2їЙТФІъЙъCl2,µ«Cl2УлNaOH·ґУ¦Ј¬±»Ч°ЦГcОьКХЈ»±ҐєНNH4ClИЬТєУлПыКЇ»Т·ґУ¦ІъЙъµДNH3»б±»cЦРµДЛ®ОьКХЎЈЦ»УРCuУлПЎHNO3·ґУ¦ІъЙъµДNOїЙТФЦЖµГЈ»НЁ№эcµДДїµДКЗіэИҐNOУлЧ°ЦГЦРµДїХЖшЅУґҐ¶шЙъіЙµДNO2ЖшМеЈ¬УГP2O5ёЙФпєуїЙµГёЙФпЎўґїѕ»µДNOЖшМеЎЈ
ґр°ёЈєЈЁ1Ј©NO
ЈЁ2Ј©ўЩC2H4 Ч°ЦГЦРГ»УРОВ¶ИјЖЈ¬ОЮ·ЁїШЦЖ·ґУ¦ОВ¶И ўЪCl2 ·ґУ¦ЙъіЙµДCl2±»cЦРµДNaOHИЬТєОьКХБЛ
ўЫNH3 ·ґУ¦ЙъіЙµДNH3±»cЦРµДH2OОьКХБЛ


 ГыРЈБ·їјѕнЖЪД©іеґМѕнПµБРґр°ё
ГыРЈБ·їјѕнЖЪД©іеґМѕнПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
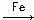
Нј15-11
ЈЁ1Ј©РґіцAЦР·ґУ¦µД»ЇС§·ЅіМКЅЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЈ?
ЈЁ2Ј©№ЫІмµЅAЦРµДПЦПуКЗЈє?ЎЎЎЎЎЎЎЎЎЎЎЎ?ЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЈ?
ЈЁ3Ј©КµСйЅбКшК±Ј¬ґтїЄAПВ¶ЛµД»оИыЈ¬ИГ·ґУ¦ТєБчИлBЦРЈ¬ід·ЦХсµґЈ¬ДїµДКЗЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЈ¬РґіцУР№ШµД»ЇС§·ЅіМКЅЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЈ?
ЈЁ4Ј©CЦРКў·ЕCCl4µДЧчУГКЗЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЈ?
ЈЁ5Ј©ДЬЦ¤Гч±ЅєНТєде·ўЙъµДКЗИЎґъ·ґУ¦Ј¬¶шІ»КЗјУіЙ·ґУ¦Ј¬їЙПтКФ№ЬDЦРјУИлAgNO3ИЬТєЈ¬ИфІъЙъµ»ЖЙ«іБµнЈ¬ФтДЬЦ¤ГчЎЈБнТ»ЦЦСйЦ¤µД·Ѕ·ЁКЗПтКФ№ЬDЦРјУИлЈ¬ПЦПуКЗЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЈ??
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
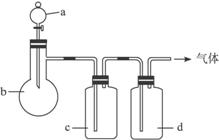
ДвУГИзНјЛщКѕЧ°ЦГЦЖИЎ±нЦРµДЛДЦЦёЙФпЎўґїѕ»µДЖшМеЈЁНјЦРМъјЬМЁЎўМъјРЎўјУИИј°ЖшМеКХјЇЧ°ЦГѕщТСВФИҐЈ»±ШТЄК±їЙТФјУИИЈ»aЎўbЎўcЎўd±нКѕПаУ¦ТЗЖчЦРјУИлµДКФјБЈ©ЎЈ
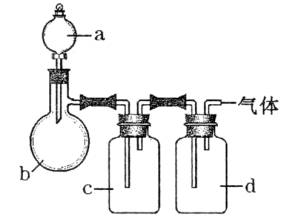
| ЖшМе | a | b | c | d |
| C2H4 | ТТґј | ЕЁH2SO4 | NaOHИЬТє | ЕЁH2SO4 |
| Cl2 | ЕЁСОЛб | MnO2 | NaOHИЬТє | ЕЁH2SO4 |
| NH3 | ±ҐєНNH4ClИЬТє | ПыКЇ»Т | H2O | №ММеNaOH |
| NO | ПЎHNO3 | НРј | H2O | P2O5 |
ЈЁ1Ј©ЙПКц·Ѕ·ЁЦРїЙТФµГµЅёЙФпЎўґїѕ»µДЖшМеКЗ____________________ЎЈ
ЈЁ2Ј©ЦёіцІ»ДЬУГЙПКц·Ѕ·ЁЦЖИЎµДЖшМеЈ¬ІўЛµГчАнУЙЎЈ
ўЩЖшМе__________Ј¬АнУЙКЗ____________________ЎЈ
ўЪЖшМе__________Ј¬АнУЙКЗ____________________ЎЈ
ўЫЖшМе__________Ј¬АнУЙКЗ____________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
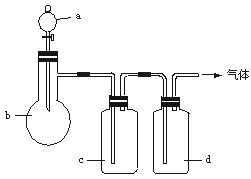
Жш Ме | a | b | c | d |
C2H4 | ТТґј | ЕЁH2SO4 | NaOHИЬТє | ЕЁH2SO4 |
Cl2 | ЕЁСОЛб | MnO2 | NaOHИЬТє | ЕЁH2SO4 |
NH3 | ±ҐєНNH4ClИЬТє | ПыКЇ»Т | H2O | №ММеNaOH |
NO | ПЎHNO3 | НРј | H2O | ЕЁH2SO4 |
ЈЁ1Ј©ЙПКц·Ѕ·ЁЦРїЙТФµГµЅёЙФпЎўґїѕ»µДЖшМеКЗ________________ЎЈ
ЈЁ2Ј©ЦёіцІ»ДЬУГЙПКц·Ѕ·ЁЦЖИЎµДЖшМеЈ¬ІўЛµГчАнУЙЈЁїЙТФІ»МоВъЈ©ЎЈ
ўЩЖшМе___________Ј¬АнУЙКЗ_________________ЎЈ
ўЪЖшМе___________Ј¬АнУЙКЗ_________________ЎЈ
ўЫЖшМе___________Ј¬АнУЙКЗ_________________ЎЈ
ўЬЖшМе___________Ј¬АнУЙКЗ_________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
Нј6-9
ЖшМе | A | b | c | d |
C2H4 | ТТґј | ЕЁH2SO4 | NaOHИЬТє | ЕЁH2SO4 |
Cl2 | ЕЁСОЛб | MnO2 | NaOHИЬТє | ЕЁH2SO4 |
NH3 | ±ҐєНNH4ClИЬТє | ПыКЇ»Т | H2O | №ММеNaOH |
NO | ПЎHNO3 | НРј | H2O | ЕЁH2SO4 |
ЈЁ1Ј©ЙПКц·Ѕ·ЁЦРїЙТФµГµЅёЙФпЎўґїѕ»µДЖшМеКЗ_______________ЈЁМо»ЇС§КЅЈ©ЎЈ
ЈЁ2Ј©ЦёіцІ»ДЬУГЙПКц·Ѕ·ЁЦЖИЎµДЖшМеЈ¬ІўЛµГчАнУЙЈЁїЙТФІ»МоВъЈ©ЎЈ
ўЩЖшМе__________Ј¬АнУЙКЗ____________________________________Ј»
ўЪЖшМе__________Ј¬АнУЙКЗ_____________________________________Ј»
ўЫЖшМе__________Ј¬АнУЙКЗ_____________________________________Ј»
ўЬЖшМе__________Ј¬АнУЙКЗ_____________________________________ЎЈ
Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com