
��������ʵ��������������õ��Ľ�����ȷ���ǣ� )
ѡ�� | ʵ����������� | ���� |
A | ���������Һ�еμ�1�η�̪��Ȼ����μ���ϡ��������ɫ��ȥ��2min���Թ���������� | �ǽ����ԣ�Cl>Si |
B | ����м��ȵ�AgNO3��Һ�еμ�KCl��Һ����Һ�ɺ�ɫ��Ϊ��ɫ | KCl��Һ���м��� |
C | ��CuSO4��Һ�еμ�KI��Һ���ټ��뱽�����а�ɫ�������ɣ��������ɫ | ��ɫ��������ΪCuI |
D | ij��Һ�μ������ữ��BaCl2��Һ�����ɰ�ɫ���� | ����Һ�п��ܲ���SO42- |
A. A B. B C. C D. D
 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ɹŰ�ͷ�и�����ѧ�ڵ�һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʽΪC7H6Cl2�ķ����廯���ﹲ��(���������칹�壩�� ��
A. 6�� B. 9�� C. 10 �� D. 12 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и�һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
����������ز�Ʒ������������Ӧ�ù㷺��
(1)���������ж����壬���ȹ�����й©ʱ������Ա������ϡ����������Һ����������__________�����û�ѧ����ʽ��ʾ����
ʵ���ҳ���NaOH��Һ���ն��������������Ҳ������������������_________������ĸ����
a.NaCl��Һ b.FeSO4��Һ c.KMnO4��Һ
(2)��ͥ�г�������Һ����Ҫ�ɷ�NaClO��������飨��Ҫ�ɷ����ᣩ�����������ijƷ������Һ��װ�ϵ�˵������ͼ��
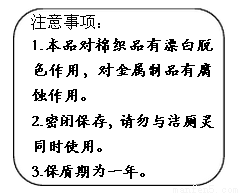
�ٷ������Խ�����Ʒ�и�ʴ���á���ԭ��_______________��
���衰�ܱձ��桱��ԭ��______________��
�ۡ�������ͬʱʹ�á�������ж�����������д����Ӧ�����ӷ���ʽ_____________��
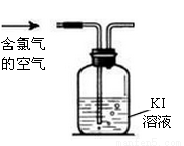
(3)��֪������������������0.1mg/m3�ͻ������ж���ijҺ������������һ�βⶨ�����������ĺ���ʱ���������0.001ml/L Kl��Һ100mL��Ϊ���жϿ����������ĺ����Ƿ꣬����Ҫ��õ�������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и�һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����Լ��ı��淽������ȷ����
A. NaOH��Һ���������в�������ϸ��ƿ��
B. ������ͨ���ܷⱣ����ú����
C. ��ˮͨ����������ɫϸ��ƿ������������
D. ����ᱣ���������Լ�ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ��ͨ�и����߿�ȫ��ģ�⣨�壩��ѧ �Ծ��������棩 ���ͣ������
��NaCl��Ϊԭ���Ʊ�KClO4�Ĺ������£�
������Ĥ�����������£�������Ӧ��NaCl+H2O��NaClO3+H2��(δ��ƽ)
����NaClO3��Һ�м���KCl�������ֽⷴӦ�����½ᾧ����KClO3��
��һ�������·�Ӧ��4KClO3��3KClO4+KCl�����������õ�KClO4��
��1�����ʱ����������Ϊ2.13g NaClO3��ͬʱ�õ�H2�����Ϊ____________L(��״��)��
��2����NaClO3��Һ�м���KCl�ܵõ�KClO3��ԭ����________________��
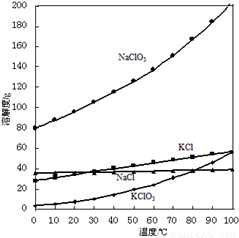
��3���ù����Ƶõ�KClO4��Ʒ�к�����KCl���ʣ�Ϊ�ⶨ��Ʒ���Ƚ�������ʵ�飺
ȷ��ȡ5.689g��Ʒ����ˮ�У����250mL��Һ������ȡ��25.00mL����ƿ�У��������������ǣ�����ʹClO4��ȫ��ת��ΪCl- (��ӦΪ��3 KClO4 +C6H12O6 �T 6 H2O + 6 CO2�� + 3 KCl������������K2CrO4��Һ��ָʾ������0. 20mol/L AgNO3��Һ���еζ����յ㣬����AgNO3��Һ���21.00mL���ζ��ﵽ�յ�ʱ������ש��ɫAg2CrO4������
�� ��֪��Ksp(AgCl)��1.8��10��10��Ksp(Ag2CrO4)��1.1��10��12����c(CrO42��)��1.1��10-4mol/L�����ʱc(Cl��)��________________mol/L��
�� ����KClO4��Ʒ�Ĵ��ȣ���д��������̡���______________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ��ͨ�и����߿�ȫ��ģ�⣨�壩��ѧ �Ծ��������棩 ���ͣ�ѡ����
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ�
��CH3OH��g��+H2O��g���TCO2��g��+3H2��g������H=��49.0kJ/mol
��CH3OH��g��+1/2O2��g���TCO2��g��+2H2��g������H=��192.9kJ/mol
����˵����ȷ����

A��CH3OH��ȼ����Ϊ192.9kJ/mol
B����Ӧ���е������仯����ͼ��ʾ
C��CH3OHת���H2�Ĺ���һ��Ҫ��������
D�����ݢ���֪��Ӧ��CH3OH��l��+1/2O2��g��=CO2��g��+2H2��g���ġ�H����192.9kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ��ͨ�и����߿�ȫ��ģ�⣨�壩��ѧ �Ծ��������棩 ���ͣ�ѡ����
��ѧ���ѧ����������ᡢ����������ء������й�˵���д�����ǣ� ��
A. �ش�����ڼ�ȼ�ŵ�������ɫ��ijЩ����Ԫ����ɫ��Ӧ�����ֳ�����ɫ��
B. С�մ���������ͷ������ȸ������ɼ���Ҳ������θ������һ��ҩ��
C. Ϊ��ֹ�±��ȸ�֬ʳƷ�����������ʣ����ڰ�װ���з�����ʯ�һ�轺
D. ����ʵʩ����ȼ�ϵ������������������Լ���SO2��NO2���ŷ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ���ݰ�һ�С�Ȫ��ʵ����ѧ��һ��ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��Һ����Mg2+��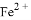 ��
��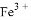 ��
��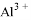 ���������ӣ��������м��������
���������ӣ��������м��������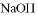 ��Һ���Ȳ����裬���ˣ����������ټ�����������ᣬ������Һ�д������ӵ��������ǣ� ��
��Һ���Ȳ����裬���ˣ����������ټ�����������ᣬ������Һ�д������ӵ��������ǣ� ��
A. 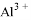 B.
B. 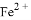 C.
C. 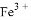 D. Mg2+
D. Mg2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2016-2017ѧ��߶���ѧ�ڵ�һ���¿���3�£���ѧ�Ծ� ���ͣ������
���仯�����ڹ�ũҵ������Ӧ�ù㷺��
��1����̬��ԭ�ӵĺ�������Ų�ʽΪ__________����_______�ֲ�ͬ�����ĵ��ӡ�
��2��BF3����ˮ����һ�������¿�ת��ΪH3O+��[B(OH)F3]-���������������ӵĿռ乹��Ϊ_____________�������ӵ�����ԭ�ӹ������________�ӻ���Cu2+Ҳ��NH3�γ������ӣ���֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2���γ������ӣ���ԭ����_________________________________________________��
��3����BH4-��Ϊ�ȵ�����ķ�����_______________(д��ѧʽ����
��4��EminBF4���۵�Ϊ12 �棬�ڳ�����ΪҺ�壬���л���������[Emin]+��[BF4]-���ɡ������ʵľ�������_________���塣
��5������ͬ�����Ga����As�γ� GaAs���壬�þ�����۵�Ϊ1238�棬�ܶ�Ϊ��g��cm-3���侧���ṹ��ͼ��ʾ���þ��������Ϊ________________��Ga��As��Ħ�������ֱ�ΪMGa g��mol-1 ��MAs g��mol-1��ԭ�Ӱ뾶�ֱ�ΪrGa pm��rAs pm������٤������ֵΪNA����GaAs������ԭ�ӵ����ռ��������İٷ���Ϊ____________________��
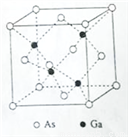
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com