
����С������9�֣� ��ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ�����Իش��������⣺
��1��ʵ��1����Ͳ�ڵ������ǣ���___________���ɣ���Ͳ����________�ƶ��������⡢���ڡ���������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��___________��Һ�С�
��2��ʵ��2�У����е�3mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������_______��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽΪ____________________��
��3��ʵ��3�У���֪��3Cl2��2NH3===N2��6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ��Ϊ____________�������Ͳ��ʣ����������ԼΪ________mL��
��1����ɫ���壻���ڣ�NaOH����ÿ��1�֣�
��2��NO ��1�֣��� 3NO2+H2O=2HNO3��NO��2�֣���
��3����ɫ��1�֣��� 5��2�֣�
����:��1��SO2��H2S����Ԫ�صĻ��ϼ۷ֱ��ǣ�4�ۺͣ�2�ۣ����Զ��߿��Է���������ԭ��Ӧ���ɵ������ˮ������ʽΪSO2��2H2S=3S��2H2O�����Է�Ӧ��ѹǿ���ͣ���Ͳ���������ƶ���SO2��H2S�����ڴ�����Ⱦ���Ҫβ�����������ü�Һ�����ա�
��2��NO2����ˮ�ķ���ʽΪ3NO2+H2O=2HNO3��NO���������յ���������ɫ��NO��
��3���������Խ������������ɵ���������������ԭ�����Ȼ��⡣���ݷ���ʽ���йط�Ӧ��������֪��15ml������������10ml����������5ml������30ml�Ȼ��⡣������ʣ��30ml�������İ������Ȼ���ǡ�÷�Ӧ�����Ȼ�泥��Ӷ����ְ�������������յ�������5ml������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����С������9�֣� ��ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ�����Իش��������⣺
��1��ʵ��1����Ͳ�ڵ������ǣ���___________���ɣ���Ͳ����________�ƶ��������⡢���ڡ���������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��___________��Һ�С�
��2��ʵ��2�У����е�3mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������_______��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽΪ____________________��
��3��ʵ��3�У���֪��3Cl2��2NH3===N2��6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ��Ϊ____________�������Ͳ��ʣ����������ԼΪ________mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�ϰ�һ�и�һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
����С������9�֣���ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ�����Իش��������⣺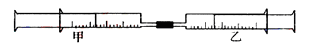

��1��ʵ��1����Ͳ�ڵ������ǣ���___________���ɣ���Ͳ����________�ƶ��������⡢���ڡ���������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��___________��Һ�С�
��2��ʵ��2�У����е�3mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������_______��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽΪ____________________��
��3��ʵ��3�У���֪��3Cl2��2NH3===N2��6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ��Ϊ____________�������Ͳ��ʣ����������ԼΪ________mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�ϰ�һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
����С������9�֣�
��1����6 mol A�����5 mol B�����Ϸ���4 L�ܱ������У���һ�������·�����Ӧ��3A(g)+B(g) 2C(g)+xD(g)����5min�ﵽƽ�⣬��ʱ����2 mol C�����D��ƽ����Ӧ����Ϊ0.15 mol/(L��min)����ƽ��ʱA�����ʵ���Ũ����____________��B��ת������__________��x��ֵ��___________��
2C(g)+xD(g)����5min�ﵽƽ�⣬��ʱ����2 mol C�����D��ƽ����Ӧ����Ϊ0.15 mol/(L��min)����ƽ��ʱA�����ʵ���Ũ����____________��B��ת������__________��x��ֵ��___________��
��2����Ӧ2H2��g�� + O2��g���� 2H2O��g���������仯��ͼ��ʾ����֪��1molH2��1molO2��1molH-O�еĻ�ѧ���ֱ���Ҫ����436KJ��496KJ��463KJ��������÷�Ӧ ������ա� �ų����� KJ������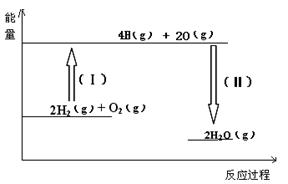
��3����������������ɴ���ʹ����һ������װ�ã��乹������ͼ��ʾ��
a��b�����缫���ɶ��̼����ɡ�д��a���ĵ缫��Ӧʽ�� ��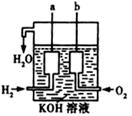
��4����ҵ���õ�ⱥ��ʳ��ˮ�ķ�����ȡ������������Ӧ��������ת����ʽ�� �������� ���ϲ�������Ӧ����Һ��pH ���������С������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
����С������9�֣�
��1����6
mol A�����5 mol B�����Ϸ���4 L�ܱ������У���һ�������·�����Ӧ��3A(g)+B(g) 2C(g)+xD(g)����5min�ﵽƽ�⣬��ʱ����2 mol C�����D��ƽ����Ӧ����Ϊ0.15 mol/(L��min)����ƽ��ʱA�����ʵ���Ũ����____________��B��ת������__________��x��ֵ��___________��
2C(g)+xD(g)����5min�ﵽƽ�⣬��ʱ����2 mol C�����D��ƽ����Ӧ����Ϊ0.15 mol/(L��min)����ƽ��ʱA�����ʵ���Ũ����____________��B��ת������__________��x��ֵ��___________��
��2����Ӧ2H2��g�� + O2 ��g���� 2H2O��g�� �������仯��ͼ��ʾ����֪��1molH2��1molO2��1molH-O�еĻ�ѧ���ֱ���Ҫ����436KJ��496KJ��463KJ��������÷�Ӧ ������ա� �ų����� KJ������

��3����������������ɴ���ʹ����һ������װ�ã��乹������ͼ��ʾ��
a��b�����缫���ɶ��̼����ɡ�д��a���ĵ缫��Ӧʽ�� ��

��4����ҵ���õ�ⱥ��ʳ��ˮ�ķ�����ȡ������������Ӧ��������ת����ʽ�� �������� ���ϲ�������Ӧ����Һ��pH �� �������С������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ��һ��һѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
����С������9�֣� ��ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ�����Իش��������⣺


��1��ʵ��1����Ͳ�ڵ������ǣ���___________���ɣ���Ͳ����________�ƶ��������⡢���ڡ���������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��___________��Һ�С�
��2��ʵ��2�У����е�3mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������_______��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽΪ____________________��
��3��ʵ��3�У���֪��3Cl2��2NH3===N2��6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ��Ϊ____________�������Ͳ��ʣ����������ԼΪ________mL��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com