
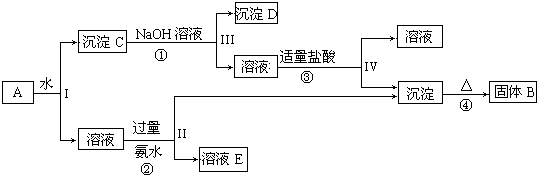
·ÖĪö Al2£ØSO4£©3ČÜÓŚĖ®£¬Al2O3ŗĶFe2O3¾ł²»ČÜÓŚĖ®£¬»ģŗĻĪļA¼ÓĖ®Čܽāŗó£¬ČÜŅŗÖŠŹĒKAl£ØSO4£©2£¬³ĮµķCĪŖAl2O3ŗĶFe2O3£»
ÓÉ×Ŗ»Æ¹ŲĻµĶ¼æÉÖŖ£¬Ļņ³ĮµķCÖŠ¼ÓNaOHČÜŅŗ£¬Fe2O3²»·“Ó¦£¬³ĮµķDĪŖFe2O3£¬Al2O3æÉÓėNaOHČÜŅŗ·“Ӧɜ³ÉNaAlO2£¬ĻņNaAlO2ČÜŅŗÖŠĶØČėCO2æɵĆAl£ØOH£©3³Įµķ£¬Al£ØOH£©3ŹÜČČ·Ö½āÉś³É¹ĢĢåBĪŖAl2O3£»
ĻņČÜŅŗÖŠ¼Ó¹żĮæ°±Ė®£¬ČÜŅŗÓė¹żĮæ°±Ė®·“Ó¦£¬Al3+±»³Įµķ£¬µĆµ½ĒāŃõ»ÆĀĮ³Įµķ£¬ČÜŅŗÖŠEĪŖ£ØNH4£©2SO4£¬¾¹żÕō·¢”¢½į¾§£¬µĆµ½£ØNH4£©2SO4£¬Č»ŗó½įŗĻĪļÖŹµÄŠŌÖŹ¼°»ÆѧÓĆÓļĄ“½ā“š£®
½ā“š ½ā£ŗAl2£ØSO4£©3ČÜÓŚĖ®£¬Al2O3ŗĶFe2O3¾ł²»ČÜÓŚĖ®£¬»ģŗĻĪļA¼ÓĖ®Čܽāŗó£¬ČÜŅŗÖŠŹĒAl2£ØSO4£©3£¬³ĮµķCĪŖAl2O3ŗĶFe2O3£»
ÓÉ×Ŗ»Æ¹ŲĻµĶ¼æÉÖŖ£¬Ļņ³ĮµķCÖŠ¼ÓNaOHČÜŅŗ£¬Fe2O3²»·“Ó¦£¬³ĮµķDĪŖFe2O3£¬Al2O3æÉÓėNaOHČÜŅŗ·“Ӧɜ³ÉNaAlO2£¬ĻņNaAlO2ČÜŅŗÖŠĶØČėCO2æɵĆAl£ØOH£©3³Įµķ£¬Al£ØOH£©3ŹÜČČ·Ö½āÉś³É¹ĢĢåBĪŖAl2O3£»
ĻņČÜŅŗÖŠ¼Ó¹żĮæ°±Ė®£¬ČÜŅŗÓė¹żĮæ°±Ė®·“Ó¦£¬Al3+±»³Įµķ£¬µĆµ½ĒāŃõ»ÆĀĮ³Įµķ£¬ČÜŅŗÖŠEĪŖ£ØNH4£©2SO4£¬¾¹żÕō·¢”¢½į¾§£¬µĆµ½£ØNH4£©2SO4£¬
£Ø1£©ČÜŅŗŗĶ³ĮµķµÄ·ÖĄėĄūÓĆ¹żĀĖ£¬¹Ź“š°øĪŖ£ŗ¹żĀĖ£»
£Ø2£©ÓÉÉĻŹö·ÖĪöæÉÖŖBĪŖAl2O3£¬CĪŖAl2O3”¢Fe2O3£¬DĪŖFe2O3 ¹Ź“š°øĪŖ£ŗAl2O3£»Fe2O3£»
£Ø3£©·“Ó¦¢ŁĪŖAl2O3+2NaOH+3H2O=2Na[Al£ØOH£©4]£¬
·“Ó¦¢ŚĪŖAl2£ØSO4£©3+6 NH3£®H2O=3£ØNH4£©2SO4+2Al£ØOH£©3”ż£¬
·“Ó¦¢ŪĪŖNa[Al£ØOH£©4]+HCl=NaCl+H2O+Al£ØOH£©3”ż£¬
·“Ó¦¢ÜĪŖ2Al£ØOH£©3$\frac{\underline{\;\;”÷\;\;}}{\;}$Al2O3+3H2O£¬
¹Ź“š°øĪŖ£ŗAl2O3+2NaOH+3H2O=2Na[Al£ØOH£©4]£»Al2£ØSO4£©3+6 NH3£®H2O=3£ØNH4£©2SO4+2Al£ØOH£©3”ż£»Na[Al£ØOH£©4]+HCl=NaCl+H2O+Al£ØOH£©3”ż£»2Al£ØOH£©3$\frac{\underline{\;\;”÷\;\;}}{\;}$Al2O3+3H2O£»
£Ø4£©ĻņNaAlO2ČÜŅŗÖŠĶØČėCO2æɵĆAl£ØOH£©3³Įµķ£¬¼“AlO2-+CO2+2H2O=Al£ØOH£©3”ż+HCO3-£¬¹Ź“š°øĪŖ£ŗAlO2-+CO2+2H2O=Al£ØOH£©3”ż+HCO3-£®
µćĘĄ ±¾Ģāæ¼²éĪŽ»śĪļµÄĶʶĻ£¬ĪŖøßĘµæ¼µć£¬×ŪŗĻæ¼²éŌŖĖŲ»ÆŗĻĪļŠŌÖŹ£¬²ąÖŲAl”¢Fe¼°Ęä»ÆŗĻĪļŠŌÖŹµÄ漲飬עŅāŃõ»ÆĀĮµÄĮ½ŠŌ£¬Ć÷Č··¢ÉśµÄ»Æѧ·“Ó¦ĪŖ½ā“šµÄ¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ź×¼×“æöĻĀ£¬17gH2O2ÖŠŗ¬¼«ŠŌ¼üµÄŹżÄæĪŖNA | |
| B£® | 1L 1mo/LµÄFe2£ØSO4£©3ČÜŅŗÖŠŗ¬ÓŠµÄĮņĖįøłĄė×ÓŹżĪŖ3NA | |
| C£® | ±ź×¼×“æöĻĀ£¬11.2LN2ŗĶH2µÄ»ģŗĻĘųĢåÖŠĖłŗ¬Ō×ÓŹżĪŖNA | |
| D£® | 5.6gFeČÜÓŚ1L 0.3moL/LĻõĖįÖŠ£¬×ŖŅʵĵē×ÓŹżĪŖ0.3NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Al2O3”śNaAlO2 | B£® | Fe”śFeCl3 | C£® | Na2O”śNa2CO3 | D£® | SiO2”śH2SiO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ńõ»Æ»¹Ō·“Ó¦ÖŠµĆŹ§µē×Ó×ÜŹżŅ»¶ØĻąµČ | |
| B£® | ÓŠµ„ÖŹ²Ī¼ÓµÄ·“Ó¦Ņ»¶ØŹĒŃõ»Æ»¹Ō·“Ó¦ | |
| C£® | µē½āÖŹČÜŅŗÖŠŅõŃōĄė×ÓµÄ×ÜŹżŅ»¶ØĻąµČ | |
| D£® | ·Ö½ā·“Ó¦Ņ»¶ØŹĒŃõ»Æ»¹Ō·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ŹµŃéŹŅĶس£ÓĆČēÓŅĶ¼ĖłŹ¾µÄ×°ÖĆĄ“ÖĘČ”°±Ęų£®»Ų“šĻĀĮŠĪŹĢā£ŗ
ŹµŃéŹŅĶس£ÓĆČēÓŅĶ¼ĖłŹ¾µÄ×°ÖĆĄ“ÖĘČ”°±Ęų£®»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ź×¼×“æöĻĀ£¬14g COĖłŗ¬ÖŹ×ÓŹżĪŖ7NA | |
| B£® | 22gijĘųĢåŗ¬·Ö×ÓŹżĪŖ0.5NA£¬ŌņĘäĦ¶ūÖŹĮæĪŖ44 | |
| C£® | ±ź×¼×“æöĻĀ£¬33.6LCH4ÖŠŗ¬HŌ×ÓŹżĪŖ6NA | |
| D£® | 1 mol Na ĶźČ«·“Ó¦Ź±£¬Ź§Č„NAøöµē×Ó |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com