
����֪��H2A��A2-�ɱ�ʾS2-��SO42-��SO32-��SiO32-��CO32-��
��1�������£���20 mL 0.2 mol��L��1 H2A��Һ�еμ�0.2 mol��L��1 NaOH��Һ���й������ʵ����仯����ͼ�����Т����H2A�������HA���������A2�����������ͼʾ��գ�
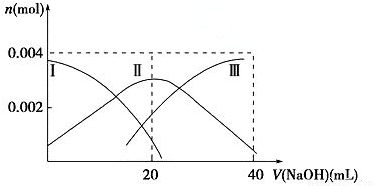
��V��NaOH����20 mLʱ����Һ������Ũ�ȴ�С��ϵ��____________��
�ڵ������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ____________�������С������ȡ�����
��2����H2AΪ���t��ʱ��pH��2��ϡ�����pH��11��NaOH��Һ�������Ϻ���Һ�����ԣ�����¶���ˮ�����ӻ�����KW��____________��
��.��֪:��25 ��ʱ H2O H++OH- KW=10-14
H++OH- KW=10-14
CH3COOH H++CH3COO- Ka=1.8��10-5
H++CH3COO- Ka=1.8��10-5
��3��������ˮ���ƽ�ⳣ��Kh������ֵ=________________,
��4��0.5 mol��L-1��������ҺpHΪm,��ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д����Ƶı�ֵ��Ϊa,1 mol��L-1��������ҺpHΪn,ˮ��ij̶�Ϊb,��m��n�Ĺ�ϵΪ___________, a��b�Ĺ�ϵΪ______________��������ڡ���С�ڡ����ڡ���
��.��5��25 ��ʱ,��a mol��L-1�İ�ˮ��b mol��L-1�����������,��Ӧ����Һǡ��������,��a��b��ʾNH3��H2O�ĵ���ƽ�ⳣ��Ϊ____________��
 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д� ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶������л�ѧ���������棩 ���ͣ�ѡ����
��4L�ܱ������г���6molA�����5molB���壬һ�������·�����Ӧ��3A��g��+B��g��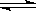 2C��g��+xD��g�����ﵽƽ��ʱ��������2molC�����ⶨ��D��Ũ��Ϊ0.5mol/L�������ж���ȷ����
2C��g��+xD��g�����ﵽƽ��ʱ��������2molC�����ⶨ��D��Ũ��Ϊ0.5mol/L�������ж���ȷ����
A��B��ת����Ϊ20%
B��ƽ��ʱA��Ũ��Ϊ1.50mol/L
C��x��1
D���ﵽƽ��ʱ������ͬ�¶��������ڻ�������ѹǿ�Ƿ�Ӧǰ��85%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶����¿�����ѧ���������棩 ���ͣ������
�����£���ijһԪ��HA���ס��ҡ�������������ͬ��һԪ�ᣩ��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ����Һ��pH���±���ʾ��
ʵ���� | HA���ʵ���Ũ��/ | NaOH���ʵ���Ũ��/ | ��Ϻ���Һ��pH |
�� | 0.1 | 0.1 | pH=a |
�� | 0.12 | 0.1 | pH=7 |
�� | 0.2 | 0.1 | pH��7 |
�� | 0.1 | 0.1 | pH=10 |
��1���Ӽ����������������ж�HA��ǿ�ỹ�����____________��
��2����������Һ������Ũ��c��A-����c��Na+���Ĵ�С��ϵ____________��
A��ǰ�ߴ� B�����ߴ� C��������� D�����ж�
��3���ӱ���ʵ�����������û����Һ������Ũ���ɴ�С��˳����____________��
��4����������ʵ�����ݣ�д�������Һ��������ʽ�ľ�ȷ�������ʽ����c��Na+��-c��A-��=_________mol•L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶����¿�����ѧ���������棩 ���ͣ�ѡ����
�������Ϊ1.0L���������ܱ������м�����������ͬ��̼�ۣ��ٷֱ����0.1 CO2��0.2molCO2���ڲ�ͬ�¶��·�ӦCO2��g����c��s�� 2CO��g���ﵽƽ�⣬ƽ��ʱCO2�����ʵ���Ũ��c��CO2�����¶ȵı仯��ͼ��ʾ��ͼ�Т������������ϣ�������˵����ȷ����
2CO��g���ﵽƽ�⣬ƽ��ʱCO2�����ʵ���Ũ��c��CO2�����¶ȵı仯��ͼ��ʾ��ͼ�Т������������ϣ�������˵����ȷ����
A����ӦCO2��g����c��s�� 2CO��g�� ��S>0����H<0
2CO��g�� ��S>0����H<0
B����ϵ����ѹǿP�ܣ�P�ܣ�״̬��>2P�ܣ�״̬��
C����ϵ��c��CO����c��CO��״̬��2c��CO��״̬��
D���淴Ӧ����V�棺V�棨״̬��>V�棨״̬��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶����¿�����ѧ���������棩 ���ͣ�ѡ����
�״����ӽ�����Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����
��CH3OH��g��+H2O��g����CO2��g��+3H2��g�� ��H�� +49.0 kJ
��CH3OH��g��+1/2O2��g����CO2��g��+2H2��g�� ��H��-192.9 kJ
����������Ӧ������˵����ȷ����
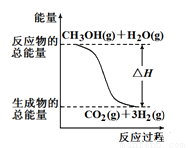
A����Ӧ���е������仯����ͼ��ʾ
B������֪2H2��g��+O2��g��=2H2O��g�� ��H��-483.8KJ/mol
C��1molCH3OH���ȼ�շų�������Ϊ192.9KJ
D��CH3OHת���H2�Ĺ���һ��Ҫ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶������л�ѧ���������棩 ���ͣ�ѡ����
������Һ�����ӵ����ʵ���Ũ�ȹ�ϵ��ȷ����
A��0.1mol��L-1NaHCO3��Һ��0.1 mol��L-1NaOH��Һ�������ϣ�������Һ�У�
c��Na������c��CO32������c��HCO3������c��OH����
B��20mL0.1 mol��L-1CH3COONa��Һ��10mL0.1 mol��L-1HCl��Һ��Ϻ�����ԣ�������Һ�У�
c��CH3COO������c��Cl������c��CH3COOH����c��H����
C�������£�pH��2��������pH��12�İ�ˮ�������ϣ�������Һ�У�
c��Cl������c��H������c��NH4������c��OH����
D��0.1 mol��L-1CH3COOH��Һ��0.1 mol��L-1NaOH��Һ�������ϣ�������Һ�У�
c��OH������c��H������c��CH3COOH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶������л�ѧ���������棩 ���ͣ�ѡ����
ijԭ����ܷ�Ӧ�����ӷ���ʽΪ��2Fe3++Fe=3Fe2+����ʵ�ָ÷�Ӧ��ԭ�����
A������Ϊͭ������Ϊ�����������ҺΪFeCl2��Һ
B������ΪC������Ϊ�����������ҺΪFe��NO3��3��Һ
C������Ϊ��������Ϊп���������ҺΪFe2��SO4��3
D������Ϊ��������Ϊ�����������ҺΪCuSO4��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶������л�ѧ���������棩 ���ͣ�ѡ����
���ڿ��淴Ӧ3H2��g��+N2��g�� 2NH3��g�������д�ʩ��ʹ��Ӧ���л���Ӱٷ�������ѧ��Ӧ���ʺͻ�ѧƽ�ⳣ�����仯����
2NH3��g�������д�ʩ��ʹ��Ӧ���л���Ӱٷ�������ѧ��Ӧ���ʺͻ�ѧƽ�ⳣ�����仯����
A������ѹǿ B�������¶� C��ʹ�ø�Ч���� D���������N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�������ĵ��������ѧ��һ�����л�ѧ���������棩 ���ͣ�ѡ����
���и�����������Һ���ܴ������棬����OH���г������ɣ�����H+���������ɵ�һ��������
A��K����Mg2����Cl����HCO B��K����Cu2����SO
B��K����Cu2����SO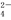 ��Na��
��Na��
C��NH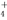 ��CO
��CO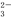 ��NO
��NO ��Na�� D��NH
��Na�� D��NH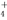 ��Cl����HCO
��Cl����HCO ��K��
��K��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com