
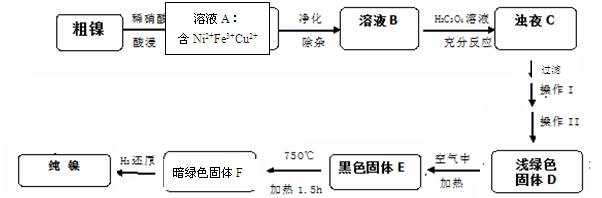
 4NiO(°µĀĢÉ«) + O2”ü
4NiO(°µĀĢÉ«) + O2”ü| ĪļÖŹ | CuS | Cu(OH)2 | Ni(OH)2 | NiS |
| Ksp | 8.8”Į10£36 | 2.2”Į10£20 | 5.48”Į10-16 | 3.2”Į10-19 |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®øĆČ¼ĮĻĀĢÉ«»·±££¬ŌŚČ¼ÉÕ¹ż³ĢÖŠ²»»įŌģ³ÉČĪŗĪ»·¾³ĪŪČ¾ |
| B£®øĆ·“Ó¦ÖŠN2O4ŹĒ»¹Ō¼Į£¬Ę«¶ž¼×ėĀŹĒŃõ»Æ¼Į |
| C£®CO2ŗĶN2¶¼ŹĒ»¹Ō²śĪļ |
| D£®ĆæÓŠ0.6molN2Éś³É£¬×ŖŅʵē×ÓŹżÄæĪŖ3.2NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 Fe3O4£«4NaOH
Fe3O4£«4NaOH| A£®øĆÉś²ś¹ż³Ģ²»»į²śÉśĪŪČ¾ | B£®·“Ó¦¢ŪÉś³ÉµÄĖÄŃõ»ÆČżĢś¾ßÓŠæ¹øÆŹ“×÷ÓĆ |
| C£®·“Ó¦¢Ł¢Ś¢Ū¾łŹĒŃõ»Æ»¹Ō·“Ó¦ | D£®·“Ó¦¢Ł¢ŚÖŠµÄŃõ»Æ¼Į¾łĪŖNaNO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā



²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®øĆŗĻ½šÖŠĶÓėĆ¾µÄĪļÖŹµÄĮæÖ®±ČŹĒ2?1 |
| B£®øĆÅØĻõĖįÖŠHNO3µÄĪļÖŹµÄĮæÅØ¶ČŹĒ14.0 mol/L |
| C£®µĆµ½2.54 g³ĮµķŹ±£¬¼ÓČėNaOHČÜŅŗµÄĢå»żŹĒ600 mL |
| D£®NO2ŗĶN2O4µÄ»ģŗĻĘųĢåÖŠ£¬NO2µÄĢå»ż·ÖŹżŹĒ80% |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ł¢Ś | B£®¢Ś¢Ū | C£®¢Ł¢Ū | D£®¢Ū¢Ü |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com