
ij������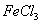 ��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ��2
��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ��2 ��
�� ��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽����
��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽����
��ȡ������Һ���μ�KSCN��Һ�Ժ�ɫ.
��ȡ10mL��Һ������������ ��Һ����������8.6l g��
��Һ����������8.6l g��
����ȡ10mL��Һ������һ��������ͭƬ����ַ�Ӧ���ͭƬ������������0��256g������Ӧ�����Һ�еμ�KSCN��Һ����ɫ��
����̽��ʵ��ó����ۣ�
(1)��Һ�к��еĽ��������� ��
(2) 10mL��Һ�������ӵ����ʵ���Ũ���� mol/L��
(3)10mL��Һ��ͭ���ӵ����ʵ����� mol��
(6��)(1)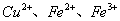 (2�֣���һ��������)
(2�֣���һ��������)
(2) 6(2��) (3) 0.006(2��)
��������
�����������1��ȡ������Һ���μ�KSCN��Һ�Ժ�ɫ��˵����Һ�к��������ӣ����ݷ�Ӧ�Ļ�ѧ����ʽ��֪����Һ�к��еĽ���������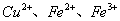 ��
��
��2��ȡ10mL��Һ������������ ��Һ����������8.6l g
��Һ����������8.6l g
���Ȼ�����������8.61g
���ʵ�����8.61g��143.5g/mol��0.06mol
����10mL��Һ�������ӵ����ʵ���Ũ����0.06mol��0.01L��6mol/L
��3���ⷨһ����0.02 ȫ����ͭ��Ӧ������Ҫͭ0.01 mol����Һ�����ܽ�0.256 gͭ(0.004mo1)��˵����Һ�к�
ȫ����ͭ��Ӧ������Ҫͭ0.01 mol����Һ�����ܽ�0.256 gͭ(0.004mo1)��˵����Һ�к� 0.006
mol��
0.006
mol��
�ⷨ����������ͭƬ��Ӧ�� Ϊ0.008 mol�������·���ϵ�ͭ��Ӧ��
Ϊ0.008 mol�������·���ϵ�ͭ��Ӧ�� Ϊ0.012 mol���������ܽ��ڷ�Һ�е�ͭΪ0.006 mol��
Ϊ0.012 mol���������ܽ��ڷ�Һ�е�ͭΪ0.006 mol��
���㣺�Ȼ�����ͭ��Ӧ���й��жϺͼ���
�����������Ǹ߿��еij������ͺͿ��㣬�����е��Ѷȵ����⡣�����ۺ���ǿ����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ�������������������Ӧ������������Ĺؼ�����ȷ��Ӧԭ����Ȼ����ݷ���ʽ��ʽ���㼴�ɡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�Ͳ�һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
ij������ ��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ��2
��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ��2 ��
�� ��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽����
��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽����
��ȡ������Һ���μ�KSCN��Һ�Ժ�ɫ.
��ȡ10mL��Һ������������ ��Һ����������8.6l g��
��Һ����������8.6l g��
����ȡ10mL��Һ������һ��������ͭƬ����ַ�Ӧ���ͭƬ������������0��256g������Ӧ�����Һ�еμ�KSCN��Һ����ɫ��
����̽��ʵ��ó����ۣ�
(1)��Һ�к��еĽ��������� ��
(2) 10mL��Һ�������ӵ����ʵ���Ũ���� mol/L��
(3)10mL��Һ��ͭ���ӵ����ʵ����� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ͼ�ѧ�����ר��ѧУ��һ���ڽ�ҵ���Ի�ѧ�Ծ����������� ���ͣ������
ij������ ��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ��
��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ�� ======
====== ��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽����
��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽����
��ȡ������Һ���μ�KSCN��Һ�Ժ�ɫ��
��ȡ10mL��Һ������������ ��Һ����������8.6l g��
��Һ����������8.6l g��
����ȡ10mL��Һ������һ��������ͭƬ����ַ�Ӧ���ͭƬ������������0��256g
����Ӧ�����Һ�еμ�KSCN��Һ����ɫ��
����̽��ʵ��ó����ۣ�
(1)��Һ�к��еĽ��������� ��
(2)�����й����ݼ���ó�ʹ�õ� ��Һ�����ʵ����ʵ���Ũ��(���踯ʴ��·�����Һ������䡣д���������)��
��Һ�����ʵ����ʵ���Ũ��(���踯ʴ��·�����Һ������䡣д���������)��
(3)10mL��Һ��ͭ���ӵ����ʵ����� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ͼ�ѧ�����ר��ѧУ��һ���ڽ�ҵ���Ի�ѧ�Ծ��������棩 ���ͣ������
ij������ ��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ��
��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ�� ======
====== ��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽����
��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽����
��ȡ������Һ���μ�KSCN��Һ�Ժ�ɫ��
��ȡ10mL��Һ������������ ��Һ����������8.6l g��
��Һ����������8.6l g��
����ȡ10mL��Һ������һ��������ͭƬ����ַ�Ӧ���ͭƬ������������0��256g
����Ӧ�����Һ�еμ�KSCN��Һ����ɫ��
����̽��ʵ��ó����ۣ�
(1)��Һ�к��еĽ��������� ��
(2)�����й����ݼ���ó�ʹ�õ� ��Һ�����ʵ����ʵ���Ũ��(���踯ʴ��·�����Һ������䡣д���������)��
��Һ�����ʵ����ʵ���Ũ��(���踯ʴ��·�����Һ������䡣д���������)��
(3)10mL��Һ��ͭ���ӵ����ʵ����� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]() (6��)ij������
(6��)ij������![]() ��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ��
��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ��![]() ======
======![]() ��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽����
��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽����
��ȡ������Һ���μ�KSCN��Һ�Ժ�ɫ��
��ȡ10mL��Һ������������![]() ��Һ����������8.6l g��
��Һ����������8.6l g��
����ȡ10mL��Һ������һ��������ͭƬ����ַ�Ӧ���ͭƬ������������0��256g
����Ӧ�����Һ�еμ�KSCN��Һ����ɫ��
����̽��ʵ��ó����ۣ�
(1)��Һ�к��еĽ��������� ��
(2)�����й����ݼ���ó�ʹ�õ�![]() ��Һ�����ʵ����ʵ���Ũ��(���踯ʴ��·�����Һ������䡣д���������)��
��Һ�����ʵ����ʵ���Ũ��(���踯ʴ��·�����Һ������䡣д���������)��
(3)10mL��Һ��ͭ���ӵ����ʵ����� mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com