
(14��)��ͼ��ʾ�ķ�Ӧ��ϵ�У����ֲ��ﱻ��ȥ����֪2mol��ɫ�����ĩ���ȷֽ⣬�ָ����������ɰ�ɫ����A.��ɫҺ��B.��ɫ����C��1mol��X.E.G����ɫ��Ӧ��Ϊ��ɫ��
�ش��������⣺
(1)д���������ʵĻ�ѧʽ��G_____________ D_____________
(2)д��G��C��Ӧ����D�Ļ�ѧ��Ӧ����ʽ��_____________ ____________________
(3)д��X��E![]() A�����ӷ���ʽ��_____________ ________________________
A�����ӷ���ʽ��_____________ ________________________
(4)д��C��![]() �μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��
�μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��
��0.2mol![]() ת�Ƶĵ�����Ϊ_____________����
ת�Ƶĵ�����Ϊ_____________����
(5)д������X����;������д��һ�֣�______________________________________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010������е���У�������Ĵθ߿�ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
(14��)��ͼ��ʾ�й�����֮���ת����ϵ������A��B��Ϊ��ɫ����Һ��B����ɫ��Ӧ�ʻ�ɫ��F���γ��������Ⱦ��֮һ��H������ˮ�Ҳ�����ϡ���ᡣ(���ֲ�����ȥ)
��1��A��B��E��F���������ʷֱ�Ϊ �� �� �� (�ѧʽ)��
��2����Ӧ�ٵ����ӷ�Ӧ����ʽΪ ��
��3����E��F�����ʵ���֮��Ϊn(E) : n(F)��1:1�����ڻ����ҺG�е��뼸��ʯ��
: n(F)��1:1�����ڻ����ҺG�е��뼸��ʯ��
��Һ���ɹ۲쵽������Ϊ ��
��4����E��F�����ʵ���֮��1:1������з��������ӷ�Ӧ����ʽΪ
��
��5�����з����ķ�Ӧ���ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ����һ�и�һ��ѧ�ڵڶ��ζο���ѧ�Ծ� ���ͣ������
(14��)��ͼ��ʾ�ķ�Ӧ��ϵ�У����ֲ��ﱻ��ȥ����֪2mol��ɫ�����ĩ���ȷֽ⣬�ָ����������ɰ�ɫ����A.��ɫҺ��B.��ɫ����C��1mol��X.E.G����ɫ��Ӧ��Ϊ��ɫ��
�ش��������⣺
(1)д���������ʵĻ�ѧʽ��G_____________ D_____________
(2)д��G��C��Ӧ����D�Ļ�ѧ��Ӧ����ʽ��_________________________________
(3)д��X��E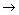 A�����ӷ���ʽ��_____________________________________
A�����ӷ���ʽ��_____________________________________
(4)д��C�� �μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��
�μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��
��0.2mol ת�Ƶĵ�����Ϊ_____________����
ת�Ƶĵ�����Ϊ_____________����
(5)д������X����;������д��һ�֣�______________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�����и�����ѧ���ڳ����Ի�ѧ�Ծ� ���ͣ������
(14��)��ͼ��ú������ҵ����һ���֣���������ѧ֪ʶ������������⣺
(1)��֪�ò�ҵ����ij��Ӧ��ƽ�����ʽΪ��K=
������Ӧ�Ļ�ѧ��ӦΪ��
(2)��֪��һ���¶��£�����Ӧ��ƽ�ⳣ�����£�
C(s)+C02(g)  2C0(g) ��H>O��K1
��
��
2C0(g) ��H>O��K1
��
��
CO(g)+H20(g)  H2(g)+C02(g)��K2 ������ ��
��
H2(g)+C02(g)��K2 ������ ��
��
C(s)+H20(g)  CO(g)+H2(g)��K3 ���������� ��
CO(g)+H2(g)��K3 ���������� ��

��K1��K2��K3��֮��Ĺ�ϵ�ǣ��������� ������������������������
��Ӧ�ٵ�ƽ�ⳣ��K���¶ȵ����߶� �� �� (����С������)��
һ���¶��£��������ݻ���Ϊ2L�������о������Ţ۷�Ӧ�������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д������Ӧ�Ŀո�

�������ɣ��������������������� ������������������������������������
(3)�ò�ҵ���а����������������ᣬ�˹������漰���������N0��N02��N204�ȡ��Է�ӦN2O4(g)
 2N02(g) ��H>O�����¶�ΪT1��T2ʱ��ƽ����ϵ��N02�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����( )
2N02(g) ��H>O�����¶�ΪT1��T2ʱ��ƽ����ϵ��N02�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����( )

A��A��C����ķ�Ӧ���ʣ�A>C
B��A��C�����������ɫ��A�Cdz
C��B��C����������ƽ����Է���������B<C
D����״̬B��״̬A�������ü��ȵķ���
(4)���(3)�еķ�Ӧ�ﵽƽ��ı�ijһ�������(���ı�N204��N02����)����Ӧ����v��ʱ��t��ϵ����ͼ��ʾ��ͼ��t4ʱ����ƽ���ƶ������������� ��ͼ�б�ʾƽ��������N02�ĺ�����ߵ�һ��ʱ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ��һ��ѧ�ڵڶ��ζο���ѧ�Ծ� ���ͣ������
(14��)��ͼ��ʾ�ķ�Ӧ��ϵ�У����ֲ��ﱻ��ȥ����֪2mol��ɫ�����ĩ���ȷֽ⣬�ָ����������ɰ�ɫ����A.��ɫҺ��B.��ɫ����C��1mol��X.E.G����ɫ��Ӧ��Ϊ��ɫ��

�ش��������⣺
(1)д���������ʵĻ�ѧʽ��G_____________ D_____________
(2)д��G��C��Ӧ����D�Ļ�ѧ��Ӧ����ʽ��_____________ ____________________
(3)д��X��E A�����ӷ���ʽ��_____________ ________________________
A�����ӷ���ʽ��_____________ ________________________
(4)д��C�� �μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��
�μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��
��0.2mol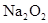 ת�Ƶĵ�����Ϊ_____________����
ת�Ƶĵ�����Ϊ_____________����
(5)д������X����;������д��һ�֣�______________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com