
| A�������£����ʵ���Ũ�Ⱦ�Ϊ0.1mol��L?1Na2CO3��NaHCO3�ĵ���������Һ�У� 2c(OH?)��2c(H+)��3c(H2CO3)��c(HCO3��)��c(CO32?) |
| B����H��0,��S��0�ķ�Ӧ�����Է���Ӧ����H��0,��S��0�ķ�Ӧ�κ��������Ƿ��Է���Ӧ�� |
| C����֪��P4(g)��6Cl2(g)��4PCl3(g) ��H��akJ��mol��1 P4(g)��10Cl2(g)��4PCl5(g)��H��bkJ��mol��1 P4������������ṹ��PCl5��P��Cl���ļ���ΪckJ��mol��1,PCl3��P��Cl���ļ���Ϊ1.2ckJ��mol��1���ɴ˼���Cl��Cl���ļ��� 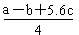 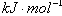 |
D����һ���¶��£��̶����Ϊ2L�ܱ������У�������Ӧ��2SO2(g)+O2(g) 2SO3(g) 2SO3(g) |
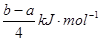 ���ɣ�
���ɣ�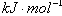 =
=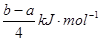 �� Q=
�� Q=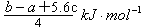 ����C����
����C����

 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ϸ�Ӧ������Ƿ��ȷ�Ӧ |
| B��������Ҫ���ȵķ�Ӧ���������ȷ�Ӧ |
| C����ϵ�Ļ��Ҷ�����ķ�Ӧ�������ȷ�Ӧ |
| D���кͷ�Ӧ�Ƿ��ȷ�Ӧ���仯ѧ�ܿ���ת���ɵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 CO(g)��3H2(g)���÷�Ӧ��H����206 kJ��mol��1��
CO(g)��3H2(g)���÷�Ӧ��H����206 kJ��mol��1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��SO2(g)�� O2(g) O2(g)  SO3(g)��H����98.3 kJ��mol��1 SO3(g)��H����98.3 kJ��mol��1 |
B��2SO2(g)��O2(g)  2SO3(l)��H����196.6 kJ��mol��1 2SO3(l)��H����196.6 kJ��mol��1 |
C��SO2(g)�� O2(g) O2(g)  SO3(g)��H����78.64 kJ��mol��1 SO3(g)��H����78.64 kJ��mol��1 |
D��2SO2(g)��O2(g)  2SO3(g)��H����196.6 kJ��mol��1 2SO3(g)��H����196.6 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��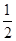 H2(g)�� H2(g)��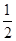 Cl2(g)=HCl(g) ��H����183 kJ��mol��1 Cl2(g)=HCl(g) ��H����183 kJ��mol��1 |
| B��H2(g)��Cl2(g)=2HCl(g)����H����183 kJ��mol��1 |
C��HCl(g)=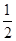 H2(g)�� H2(g)��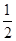 Cl2(g)��H����91.5 kJ��mol��1 Cl2(g)��H����91.5 kJ��mol��1 |
| D��H2(g)��Cl2(g)=2HCl(g)����H����248 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 H2O(l)����H="-57.3" kJ��mol-1,�ش��������⡣
H2O(l)����H="-57.3" kJ��mol-1,�ش��������⡣�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
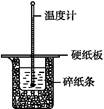
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
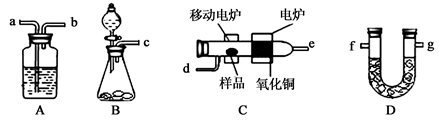
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com