
| A��24n��A1��+35��5n��Na��=W2��W1 | B��n��Na��+3n��A1��=aV2 |
| C��n��Na��+3n��A1��=V1/11��2 | D��aV2=V1/22��4 |


 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na+��Mg2+ | B��Al3+��Mg2+ | C��Mg2+��AlO2- | D��Na+��AlO2- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��þԭ�ӵļ۵����������� |
| B��þ����Ũ��������ᣬ���治�ܶۻ����������� |
| C��þ�����ڿ����к���ʢCO2�ļ���ƿ��ȼ�գ������������� |
| D������Ӳ�ȡ���չ�ԡ��۵����þ�á��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
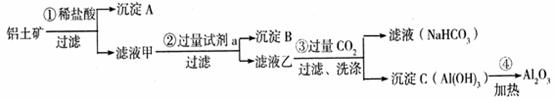
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
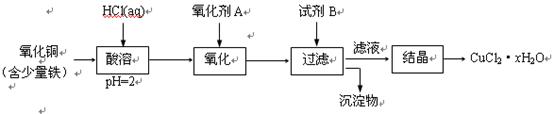
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ����������V����ʱ�䣨t����ϵ��ͼ����Ӧ��þ������
����������V����ʱ�䣨t����ϵ��ͼ����Ӧ��þ������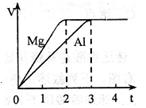

| A�����ʵ���֮��Ϊ3��2 | B������֮��Ϊ3��2 | C��Ħ������֮��Ϊ2��3 | D����Ӧ����֮��Ϊ2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 + H2O ��C+ NaOH��B+NaCl ��E+ H2O��NaOH+F
+ H2O ��C+ NaOH��B+NaCl ��E+ H2O��NaOH+F ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
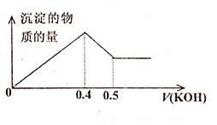
| A��1��3 | B��2��3 | C��3��1 | D��6��1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com