
ijŠ”×éĶ¬Ń§ÓūŃŠ¾æSO2µÄŠŌÖŹ”£
(1)½«Ļą¹ŲµÄŗ¬ĮņĪļÖŹ·ÖĪŖČēĻĀ±ķĖłŹ¾3×飬µŚ2×éÖŠĪļÖŹXµÄ»ÆѧŹ½ŹĒ ”£
| µŚ1×é | µŚ2×é | µŚ3×é |
| S(µ„ÖŹ) | SO2”¢X”¢Na2SO3”¢NaHSO3 | SO3”¢H2SO4”¢Na2SO4”¢NaHSO4 |

(1)H2SO3 (2)¢Ł2SO2£«O2 2SO3
2SO3
¢ŚŹ¹SO3Äż½į³É¹ĢĢåÓėSO2·ÖĄė 5SO2£«2H2O£«2MnO4”Ŗ£½5SO42”Ŗ£«2Mn2£«£«4H£«
¢Ū3SO2£«4NaOH£½Na2SO3£«2NaHSO3£«H2O
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©½«Ļą¹ŲµÄŗ¬ĮņĪļÖŹŅĄ¾ŻĮņŌŖĖŲ»ÆŗĻ¼Ū·Ö×飬ŅĄ¾ŻµĶČż×å·ÖĄąĖ³Šņ£¬ÅŠ¶ĻXĪŖ+4¼ŪµÄĖįĪŖH2SO3 £»
¹Ź“š°øĪŖ£ŗH2SO3 £»
£Ø2£©¢Ł¹¤ŅµÉś²śÖŠSO2“ß»ÆŃõ»ÆµÄ·“Ó¦ŹĒ¶žŃõ»ÆĮņµÄ“ß»ÆŃõ»Æ·“Ó¦£»»Æѧ·½³ĢŹ½ĪŖ£ŗ2SO2+O2 2SO3£»
2SO3£»
¹Ź“š°øĪŖ£»2SO2+O2 2SO3£»
2SO3£»
¢Ś×°ÖĆIIµÄ×÷ÓĆŹĒĄäȓװÖĆ£¬ČżŃõ»ÆĮņµÄ·Šµć½ĻµĶ£¬Ķعż±łĖ®»ģŗĻĪļ£¬Ź¹SO3Äż½į³É¹ĢĢåÓėSO2·ÖĄė£¬×°ÖĆIIIÖŠøßĆĢĖį¼ŲČÜŅŗ»įŗĶ¶žŃõ»ÆĮņ·“Ó¦Öš½„ĶŹÉ«£¬Éś³ÉMn2+£¬¶žŃõ»ÆĮņ±»Ńõ»ÆĪŖĮņĖį£»
·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ5SO2+2H2O+2MnO4=5SO42-+2Mn2++4H+£»
¹Ź“š°øĪŖ£ŗŹ¹SO3Äż½į³É¹ĢĢåÓėSO2·ÖĄė£¬5SO2+2H2O+2MnO4=5SO42-+2Mn2++4H+£»
¢ŪČō×°ÖĆIVÖŠÓŠ40mL 2.5mol?L-1NaOHČÜŅŗ£¬·“Ó¦ŗóŌöÖŲ4.8g£¬ĪüŹÕµÄĪŖ¶žŃõ»ÆĮņĘųĢ壬ŅĄ¾Ż¶žŃõ»ÆĮņĪļÖŹµÄĮæŗĶĒāŃõ»ÆÄĘĪļÖŹµÄĮæ½ųŠŠ·ÖĪöÅŠ¶ĻÉś³É²śĪļ£¬Čōn£ØSO2£©£ŗn£ØNaOH£©=1£ŗ2·“Ó¦°“ÕÕ·“Ó¦£ŗSO2+2NaOH=Na2SO3+H2O£»Čōn£ØSO2£©£ŗn£ØNaOH£©=1£ŗ1£¬·“Ó¦°“ÕÕ·“Ó¦SO2+NaOH=NaHSO3£»×°ÖĆIVÖŠÓŠ40mL 2.5mol?L-1NaOHČÜŅŗÖŠn£ØNaOH£©=0.1mol£¬n£ØSO2£©="4.8g”Ā64g/mol" =0.075mol£»n£ØSO2£©£ŗn£ØNaOH£©=0.075£ŗ0.1=3£ŗ4£¬ĖłŅŌÉś³É²śĪļĪŖŃĒĮņĖįÄĘŗĶŃĒĮņĖįĒāÄĘ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŅĄ¾ŻÖ±Ę½·ØŠ“³ö£ŗ3SO2+4NaOHØTNa2SO3+2NaHSO3+H2O£»
¹Ź“š°øĪŖ£ŗ3SO2+4NaOHØTNa2SO3+2NaHSO3+H2O”£
æ¼µć£ŗ¶žŃõ»ÆĮņµÄ»ÆѧŠŌÖŹ
µćĘĄ£ŗ±¾Ģāæ¼²éĮĖĪļÖŹ·ÖĄą·½·Ø£¬¹ęĀÉ×ܽį£¬ŹµŃé¹ż³Ģ·ÖĪöÅŠ¶Ļ£¬»Æѧ·½³ĢŹ½ŗĶĄė×Ó·½³ĢŹ½µÄŹéŠ“£¬·“Ó¦²śĪļµÄ·ÖĪöÅŠ¶Ļ£¬ĢāÄæÄѶČÖŠµČ”£


 ŠĀ¾ķĶõĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø
ŠĀ¾ķĶõĘŚÄ©³å“Ģ100·ÖĻµĮŠ“š°ø Č«ÄÜ“³¹Ų100·ÖĻµĮŠ“š°ø
Č«ÄÜ“³¹Ų100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ”³¤É³ŹŠøßČżµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©Ä³»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§½ųŠŠCl2”¢NH3µÄÖʱø¼°ŠŌÖŹ¼ģŃéµČŹµŃéµÄĮ÷³ĢŗĶ²æ·Ö×°ÖĆČēĻĀ£ŗ
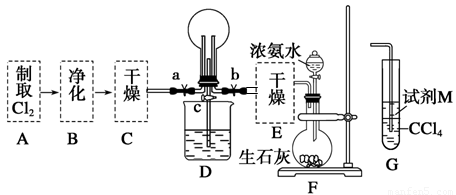
ĒėĄūÓĆA”¢G×°ÖĆÉč¼ĘŅ»øö¼ņµ„µÄŹµŃéŃéÖ¤Cl2”¢Fe3£«”¢I2µÄŃõ»ÆŠŌĒæČõĪŖCl2>Fe3£«>I2(ŹµŃéÖŠ²»¶ĻµŲŠ”ŠÄÕńµ“G×°ÖĆÖŠµÄŹŌ¹Ü)”£AÖŠ·“Ó¦ĪļŹĒKMnO4ŗĶÅØŃĪĖį£¬ĒėŠ“³öAÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ £¬ĒėŠ“³öŹŌ¼ĮMĪŖ ČÜŅŗ£¬Ö¤Ć÷Ńõ»ÆŠŌĪŖCl2>Fe3£«>I2µÄŹµŃéĻÖĻóŹĒ ”£
¢ŚŅŃÖŖ3Cl2£«2NH3=6HCl£«N2£¬µ±DµÄÉÕĘæÖŠ³äĀś»ĘĀĢÉ«ĘųĢåŗ󣬹Ų±Õa”¢c“ņæŖb£¬DÖŠµÄĻÖĻóĪŖ»ĘĀĢÉ«ĘųĢåĻūŹ§£¬²śÉś°×ŃĢ£¬·“Ó¦Ņ»¶ĪŹ±¼äŗ󣬹Ų±Õb“ņæŖc£¬¹Ū²ģµ½µÄĻÖĻóĪŖ_________________________________________________________________”£
£Ø2£©Ä³·ĻĖ®ÖŠŗ¬ÓŠŅ»¶ØĮæµÄNa+”¢SO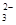 £¬æÉÄÜŗ¬ÓŠCO
£¬æÉÄÜŗ¬ÓŠCO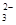 £¬Ä³ŃŠ¾æŠ”×éÓū²ā¶ØĘäÖŠSO
£¬Ä³ŃŠ¾æŠ”×éÓū²ā¶ØĘäÖŠSO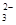 µÄÅØ¶Č£¬Éč¼ĘČēĻĀŹµŃé·½°ø£ŗ
µÄÅØ¶Č£¬Éč¼ĘČēĻĀŹµŃé·½°ø£ŗ

¢Ł“ÓĻĀĮŠŹŌ¼ĮÖŠŃ”ŌńŹŌ¼ĮXĪŖ_________£ØĢīŠņŗÅ£©£»
A£®0.1 mol/L KMnO4(H2SO4Ėį»Æ)ČÜŅŗ B£®0.5 mol/L NaOHČÜŅŗ
C£®ŠĀÖĘĀČĖ® D£®KIČÜŅŗ
¢Ś¼ÓČėŹŌ¼ĮXÉś³ÉSO µÄĄė×Ó·½³ĢŹ½ĪŖ_____________________________________£»
µÄĄė×Ó·½³ĢŹ½ĪŖ_____________________________________£»
¢ŪÖ¤Ć÷øĆ·ĻĖ®ÖŠŹĒ·ńŗ¬ÓŠCO µÄŹµŃé·½°øĪŖ
ӣ
µÄŹµŃé·½°øĪŖ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ”³¤É³ŹŠøßČżµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©Ä³»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§½ųŠŠCl2”¢NH3µÄÖʱø¼°ŠŌÖŹ¼ģŃéµČŹµŃéµÄĮ÷³ĢŗĶ²æ·Ö×°ÖĆČēĻĀ£ŗ
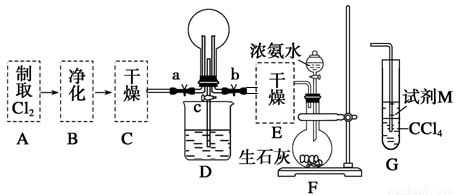
ĒėĄūÓĆA”¢G×°ÖĆÉč¼ĘŅ»øö¼ņµ„µÄŹµŃéŃéÖ¤Cl2”¢Fe3£«”¢I2µÄŃõ»ÆŠŌĒæČõĪŖCl2>Fe3£«>I2(ŹµŃéÖŠ²»¶ĻµŲŠ”ŠÄÕńµ“G×°ÖĆÖŠµÄŹŌ¹Ü)”£AÖŠ·“Ó¦ĪļŹĒKMnO4ŗĶÅØŃĪĖį£¬ĒėŠ“³öAÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ £¬ĒėŠ“³öŹŌ¼ĮMĪŖ ČÜŅŗ£¬Ö¤Ć÷Ńõ»ÆŠŌĪŖCl2>Fe3£«>I2µÄŹµŃéĻÖĻóŹĒ ”£
¢ŚŅŃÖŖ3Cl2£«2NH3=6HCl£«N2£¬µ±DµÄÉÕĘæÖŠ³äĀś»ĘĀĢÉ«ĘųĢåŗ󣬹Ų±Õa”¢c“ņæŖb£¬DÖŠµÄĻÖĻóĪŖ»ĘĀĢÉ«ĘųĢåĻūŹ§£¬²śÉś°×ŃĢ£¬·“Ó¦Ņ»¶ĪŹ±¼äŗ󣬹Ų±Õb“ņæŖc£¬¹Ū²ģµ½µÄĻÖĻóĪŖ_________________________________________________________________”£
£Ø2£©Ä³·ĻĖ®ÖŠŗ¬ÓŠŅ»¶ØĮæµÄNa+”¢SO £¬æÉÄÜŗ¬ÓŠCO
£¬æÉÄÜŗ¬ÓŠCO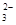 £¬Ä³ŃŠ¾æŠ”×éÓū²ā¶ØĘäÖŠSO
£¬Ä³ŃŠ¾æŠ”×éÓū²ā¶ØĘäÖŠSO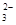 µÄÅØ¶Č£¬Éč¼ĘČēĻĀŹµŃé·½°ø£ŗ
µÄÅØ¶Č£¬Éč¼ĘČēĻĀŹµŃé·½°ø£ŗ

¢Ł“ÓĻĀĮŠŹŌ¼ĮÖŠŃ”ŌńŹŌ¼ĮXĪŖ_________£ØĢīŠņŗÅ£©£»
A£®0.1 mol/L KMnO4(H2SO4Ėį»Æ)ČÜŅŗ B£®0.5 mol/L NaOHČÜŅŗ
C£®ŠĀÖĘĀČĖ® D£®KIČÜŅŗ
¢Ś¼ÓČėŹŌ¼ĮXÉś³ÉSO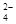 µÄĄė×Ó·½³ĢŹ½ĪŖ_____________________________________£»
µÄĄė×Ó·½³ĢŹ½ĪŖ_____________________________________£»
¢ŪÖ¤Ć÷øĆ·ĻĖ®ÖŠŹĒ·ńŗ¬ÓŠCO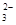 µÄŹµŃé·½°øĪŖ
ӣ
µÄŹµŃé·½°øĪŖ
ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com