
(8��)�������ʵ�����A��B�����2L���ܱ������У��������·�Ӧ��
3A(g)��B(g) xC(g)��2D(g)����5min���D��Ũ��Ϊ0.5mol��L��1��C��ƽ����Ӧ������0.1mol/(L��min)���Ҵ�ʱA��B�����ʵ���Ũ��֮��Ϊ3��5����
xC(g)��2D(g)����5min���D��Ũ��Ϊ0.5mol��L��1��C��ƽ����Ӧ������0.1mol/(L��min)���Ҵ�ʱA��B�����ʵ���Ũ��֮��Ϊ3��5����
��1����ʱA��Ũ��Ϊ .��Ӧ��ʼǰ����������A��B�����ʵ���
��2��B��ƽ����Ӧ���� .
��3��x��ֵ�Ƕ��� .
��4������������֤���÷�Ӧ�Ѵﵽ��ѧƽ��״̬���� ��
A��������ѹǿ���ٷ����仯
B��A������������ٷ����仯
C����������������������ٷ����仯
D����ͬʱ��������3nmolA��ͬʱ����nmolB
E. ��������ƽ����Է����������ٱ仯

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)������(HNO2)��һ�ֱȴ�����ǿ�����ᣬ�ܲ��ȶ�����������������ԭ��Ӧ���ֽ⡣
(1)�����£��������ʵ�����NO��NO2ͨ��ˮ�У����Ƶ�HNO2����Ӧ�Ļ�ѧ����ʽΪ__________________________________________________________.
(2)NO�������������л�ԭ�ԣ������������ԭ��������Һ��pH�Ĺ�ϵ���±���ʾ��
| pH��Χ | ����7 | С��7 |
| ���� | NO | NO��N2O��N2�е�һ�� |
���ڼ��������£�NaNO2��Һ��NaClO��Һ��Ӧ�����ӷ���ʽΪ
_________________________________________________________.
����HNO2������ᷴӦʱ�����ʵ���֮��1��2���У���I����������I2��������к���������Ϊ__________(�ѧʽ).
(3)���䶳��NaNO2��Һ�м����ͨ���������ʣ������Ƶ�HNO2����________(�����).
a��ϡH2SO4���� b��ϡHCl c��CO2 d��SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��) ��֪3A(g)��B(g) xC(g)��2D(g)���������ʵ�����A��B�����2 L���ܱ������У���5 min��ﵽ��ѧƽ�⣬��ʱ���D��Ũ��Ϊ0.5 mol/L����c(A)��c(B)��3��5����֪5min ����C��ʾ��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
��1����Ӧ��ʼǰ�����е�A��B�����ʵ�����n(A)��n(B)��________mol��
��2��5min����B��ʾ��ƽ����Ӧ����v(B)��________mol/(L��min)��
��3����ʱA��ת����Ϊ_______��x��ֵΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������ʡ�߶���ѧ���ڳ����Ի�ѧ�Ծ��������棩 ���ͣ������
(8��)�������ʵ�����A��B�����2L���ܱ������У��������·�Ӧ��
3A(g)��B(g) xC(g)��2D(g)����5min���D��Ũ��Ϊ0.5mol��L��1��C��ƽ����Ӧ������0.1mol/(L��min)���Ҵ�ʱA��B�����ʵ���Ũ��֮��Ϊ3��5����
xC(g)��2D(g)����5min���D��Ũ��Ϊ0.5mol��L��1��C��ƽ����Ӧ������0.1mol/(L��min)���Ҵ�ʱA��B�����ʵ���Ũ��֮��Ϊ3��5����
��1����ʱA��Ũ��Ϊ .��Ӧ��ʼǰ����������A��B�����ʵ���
��2��B��ƽ����Ӧ���� .
��3��x��ֵ�Ƕ��� .
��4������������֤���÷�Ӧ�Ѵﵽ��ѧƽ��״̬���� ��
A��������ѹǿ���ٷ����仯
B��A������������ٷ����仯
C������������ �����������ٷ����仯
D����ͬʱ��������3nmolA��ͬʱ����nmolB
E. ��������ƽ����Է����������ٱ仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����и����������¿���ѧ�Ծ��������棩 ���ͣ������
(8��)������(HNO2)��һ�ֱȴ�����ǿ�����ᣬ�ܲ��ȶ�����������������ԭ��Ӧ���ֽ⡣
(1)�����£��������ʵ�����NO��NO2ͨ��ˮ�У����Ƶ�HNO2����Ӧ�Ļ�ѧ����ʽΪ__________________________________________________________.
(2)NO�������������л�ԭ�ԣ������������ԭ��������Һ��pH�Ĺ�ϵ���±���ʾ��
|
pH��Χ |
����7 |
��7 |
|
���� |
NO |
NO��N2O��N2�е�һ�� |
���ڼ��������£�NaNO2��Һ��NaClO��Һ��Ӧ�����ӷ���ʽΪ
_________________________________________________________.
����HNO2������ᷴӦʱ�����ʵ���֮��1��2���У���I����������I2��������к���������Ϊ__________(�ѧʽ).
(3)���䶳��NaNO2��Һ�м����ͨ���������ʣ������Ƶ�HNO2����________(�����).
a��ϡH2SO4���� b��ϡHCl c��CO2 d��SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(8��)�������ʵ�����A��B�����2 L���ܱ������У��������·�Ӧ��
3A(g)��B(g) 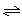 xC(g)��2D(g)����5 min���ƽ��״̬����ʱ���D��Ũ��Ϊ0.5 mol/L���������������Ϊ134.4 L����״������C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)������
xC(g)��2D(g)����5 min���ƽ��״̬����ʱ���D��Ũ��Ϊ0.5 mol/L���������������Ϊ134.4 L����״������C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)������
��1����ʱA��Ũ��c(A)��________mol/L��x��ֵΪ________��
��2����Ӧ��ʼǰ�����е�A��B�����ʵ�����n(A)��n(B)��________mol��
��3��ƽ��ʱn(A)�� n(B)�� n (C)�� n(D) =
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com