
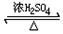 ��CH3��2CHCH2COOCH2CH2CH��CH3��2+H2O��
��CH3��2CHCH2COOCH2CH2CH��CH3��2+H2O��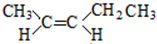 ��
��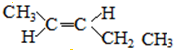 ��
�� ��
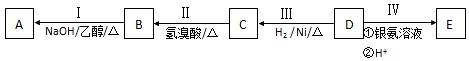
�� ���� �л���D������ͼ��������Է�������Ϊ86��8.6gD���л�������ʵ���Ϊ��n=$\frac{m}{M}$=$\frac{8.6g}{86g/mol}$=0.1mol��Ũ�������յ���ˮ����ʯ�����յ��Ƕ�����̼��ˮ�����ʵ���=$\frac{9g}{18g/mol}$=0.5mol��������̼�����ʵ���=$\frac{22g}{44g/mol}$=0.5mol�������л��������̼��ˮ�����ʵ���֮��=0.1mol��0.5mol��0.5mol=1��5��5�����Ը��л�������к���5��̼ԭ�ӡ�10����ԭ�ӣ����л������Է���������86��86-5��12-10=16�����Ը÷����л�����1����ԭ�ӣ������ʽΪ��C5H10O��D���Ľṹ�к���2��-CH3�����ĺ˴Ź��������г���4���壬˵�����л����к�4�����͵���ԭ�ӣ���DΪ��CH3��2CHCH2CHO��DΪȩ����ת����ϵ��֪��EΪ���ᡢCΪ����BΪ±������B����NaOH�Ҵ���Һ���ȵ������·�Ӧ����A��Bת��ΪA������ȥ��Ӧ����AΪϩ��������C=C��B��D��E�Ľṹ�о�����2��-CH3�����ǵĺ˴Ź��������о�����4���壬��CΪ��CH3��2CHCH2CH2OH��BΪ��CH3��2CHCH2CH2Br��AΪ��CH3��2CHCH=CH2��EΪ��CH3��2CHCH2COOH���ݴ˽��
��� �⣺��1���л���D������ͼ��������Է�������Ϊ86��8.6gD���л�������ʵ���Ϊ��n=$\frac{m}{M}$=$\frac{8.6g}{86g/mol}$=0.1mol��Ũ�������յ���ˮ����ʯ�����յ��Ƕ�����̼��ˮ�����ʵ���=$\frac{9g}{18g/mol}$=0.5mol��������̼�����ʵ���=$\frac{22g}{44g/mol}$=0.5mol�������л��������̼��ˮ�����ʵ���֮��=0.1mol��0.5mol��0.5mol=1��5��5�����Ը��л�������к���5��̼ԭ�ӡ�10����ԭ�ӣ����л������Է���������86��86-5��12-10=16�����Ը÷����л�����1����ԭ�ӣ������ʽΪ��C5H10O��D���Ľṹ�к���2��-CH3�����ĺ˴Ź��������г���4���壬˵�����л����к�4�����͵���ԭ�ӣ���DΪ��CH3��2CHCH2CHO����ת����ϵ��֪��EΪ���ᡢCΪ����BΪ±������B����NaOH�Ҵ���Һ���ȵ������·�Ӧ����A��Bת��ΪA������ȥ��Ӧ����AΪϩ��������C=C��B��D��E�Ľṹ�о�����2��-CH3�����ǵĺ˴Ź��������о�����4���壬��CΪ��CH3��2CHCH2CH2OH��BΪ��CH3��2CHCH2CH2Br�����еĹ�����Ϊ��ԭ�ӣ�D�Ľṹ��ʽΪ����CH3��2CHCH2CHO������ʽΪC5H10O��
�ʴ�Ϊ����ԭ�ӣ�C5H10O��
��2����Ӧ��Ϊȩת��Ϊ������ӦΪ����CH3��2CHCH2CHO+H2$��_{��}^{Ni}$��CH3��2CHCH2CH2OH�����ڼӳɷ�Ӧ���������ӳɷ�Ӧ���ɴ���ҲΪ��ԭ��Ӧ������a��b����������
�ʴ�Ϊ��a��b��
��3����Ӧ��Ϊ��CH3��2CHCH2CH2Br���Ҵ���������NaOH��Ӧ����ϩ����Ϊ��ȥ��Ӧ������ʽΪ��CH3��2CHCH2CH2Br+NaOH$��_{��}^{�Ҵ�}$��CH3��2CHCH=CH2+NaBr+H2O����Ӧ��Ϊ��CH3��2CHCH2CHO�������Լ�������������李�����������ˮ��Ϊ������Ӧ����Ӧ�ķ���ʽΪ����CH3��2CHCH2CHO+2Ag��NH3��2OH$��_{H+}^{ˮԡ}$��CH3��2CHCH2COOH+2Ag��+4NH3��+H2O��
�ʴ�Ϊ����CH3��2CHCH2CH2Br+NaOH$��_{��}^{�Ҵ�}$��CH3��2CHCH=CH2+NaBr+H2O����CH3��2CHCH2CHO+2Ag��NH3��2OH$��_{H+}^{ˮԡ}$��CH3��2CHCH2COOH+2Ag��+4NH3��+H2O��
��4��CΪ��CH3��2CHCH2CH2OH��EΪ��CH3��2CHCH2COOH����һ�������·�Ӧ����F��FΪ����ζ���л������FΪ�������Է�ӦΪ����CH3��2CHCH2CH2OH+��CH3��2CHCH2COOH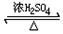 ��CH3��2CHCH2COOCH2CH2CH��CH3��2+H2O��
��CH3��2CHCH2COOCH2CH2CH��CH3��2+H2O��
�ʴ�Ϊ����CH3��2CHCH2CH2OH+��CH3��2CHCH2COOH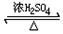 ��CH3��2CHCH2COOCH2CH2CH��CH3��2+H2O��
��CH3��2CHCH2COOCH2CH2CH��CH3��2+H2O��
��5��A��ͬ���칹������һ�Ի�Ϊ˳���칹���ҽṹ����2��-CH3�������ǵĽṹ��ʽΪ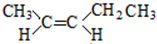 ��
��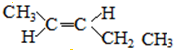 ��
��
�ʴ�Ϊ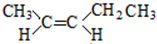 ��
��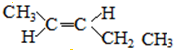 ��
��
��6��EΪ��CH3��2CHCH2COOH��E��һ��ͬ���칹���ܷ���������Ӧ������������������������������-CHO��-OH���Ҳ��ܷ�����ȥ��Ӧ����ṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϣ��漰±����������ȩ�������������ת��������ȷ��D�Ľṹ��ʽ�ǹؼ���ע���������չ����ŵ�������ת��������������ѧ�����Ӧ����ѧ֪ʶ����������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5Cl2+I2+6H2O�T10HCl+2HIO3 | |
| B�� | 2Cl2+2Ca��OH��2�TCaCl2+Ca��ClO��+2H2O | |
| C�� | MnO2+4HCI$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+2H2O+Cl2�� | |
| D�� | 2NaCl+2H2O $\frac{\underline{\;���\;}}{\;}$2NaOH++Cl2��+H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | CO2 | CO | |||
| A | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
| B | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
| C | 900 | a | b | c | d | t |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SiO2���ɼ��Լ����ɵķǼ��Է��� | |
| B�� | ��NA��ʾ����٤�����������³�ѹ��60g SiO2�к��еĹ��ۼ���ĿΪ2NA | |
| C�� | ̼��ͬ���壬���CO2��SiO2��ѧ�������ƣ���������Ҳ���� | |
| D�� | SiO2����ṹ�е���С��Ϊ6��Si��6��O��ɵ�12Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ | |
| B�� | ��ѧ�仯������ԭ�ӵ�������Ϲ��� | |
| C�� | ���������������ڷ�Ӧ���������ķ�Ӧ�����ȷ�Ӧ | |
| D�� | ��Ӧ�Ƿ��Ȼ������ȱ��뿴��Ӧ��������������е�����������Դ�С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶���10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�� Ũ��Ϊ0.1 mol��L��1 HF
Ũ��Ϊ0.1 mol��L��1 HF ��Һ��ˮ����ϡ�ͣ����и�
��Һ��ˮ����ϡ�ͣ����и� ��ʼ�ձ����������( )
��ʼ�ձ����������( )
A��c(H��) B��Ka(HF)
C��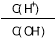 D��
D��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶���10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʣ���ǿ����ʵ��ǣ� ��
A�����ᱵ B��ʯī C��Ũ���� D��HF
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com