
�Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ٽ���Һ����ͼ��ʾ������в�����
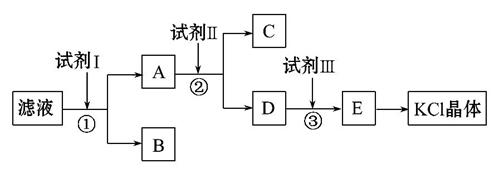
�ش��������⣺
��1����ʼ��Һ��pH__________7(����ڡ���С�ڡ����ڡ�)����ԭ����______________________________________________��
��2���Լ���Ļ�ѧʽΪ______________�����з�����Ӧ�����ӷ���ʽΪ______
__________________________________________________________________��
��3���Լ���Ļ�ѧʽΪ______________�����м����Լ����Ŀ����__________
__________________________________________________________________��
��4���Լ����������__________�����з�����Ӧ�����ӷ���ʽΪ___________
__________________________________________________________________��
��5��ijͬѧ��ȡ�ᴿ�IJ�Ʒ0.775 9 g���ܽ������100 mL����ƿ�У�ÿ��ȡ25.00 mL��Һ����0.100 0 mol��L-1������������Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ25.62 mL���ò�Ʒ�Ĵ���Ϊ________(��ʽ��������)��
.����������1����ʼ��Һ�к���̼��أ�̼���ˮ��ʼ��ԣ�����Һ��pH����7��
��2��Ҫ��������������������Ӻ�̼������ӣ�Ӧ��������ı����ӣ��ֲ��������������ӣ�����Լ���ѡ��BaCl2��Һ����S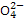 ��C
��C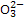 ���ܷ�Ӧ��
���ܷ�Ӧ��
��3��Ҫ��������ı����ӣ������Լ�������ܳ�ȥ������Ba2+���ֲ����������������ҹ������ӽ��׳�ȥ�����ѡ��K2CO3��
��4��Ҫ���������̼������ӣ�Ҫ�μӹ��������ᡣ
��5��������Ʒ�Ĵ��ȣ�ע��0.775 9 g��Ʒ���100 mL��Һ��ÿ��ֻȡ
25.00 mL������ӱ����������������豣����λ��Ч���֡�
�𰸣���1�����ڡ�ǿ��������K2CO3����ˮ��ʹ��ϵ�ʼ���
��2��BaCl2��S +Ba2+====BaSO4����C
+Ba2+====BaSO4����C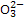 +Ba2+====BaCO3��
+Ba2+====BaCO3��
��3��K2CO3����ȥA�е�Ba2+
��4�����ᡡC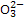 +2H+====CO2��+H2O
+2H+====CO2��+H2O
��5��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϡ�ij�о���ѧϰС�����λͬѧ��Ϊ�ⶨij��þ3%��5%����þ�Ͻ�(��������Ԫ��)��þ����������������������ֲ�ͬʵ�鷽������̽������д���пհס�
̽��һ
ʵ�鷽������þ�Ͻ� �ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��____________________________________________________________________________________________________________��
�ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��____________________________________________________________________________________________________________��
ʵ�鲽�裺
(1)��ȡ5.4 g��þ�Ͻ��ĩ��Ʒ��Ͷ��V mL 2.0 mol/L NaOH��Һ�У���ַ�Ӧ��NaOH��Һ�����V��________��
(2)���ˡ�ϴ�ӡ�����������塣�ò�������δϴ�ӹ��壬���þ������������________(�ƫ�ߡ���ƫ�͡�)��
̽����
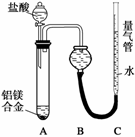
ʵ�鷽������þ�Ͻ�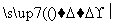 �ⶨ������������(ʵ��װ����ͼ��ʾ)���������ۣ�
�ⶨ������������(ʵ��װ����ͼ��ʾ)���������ۣ�
(1)ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��A��B֮������һ�������������װ�á���������________(���Ҫ������Ҫ��)��
(2)Ϊʹ�ⶨ��������ܾ�ȷ��ʵ����Ӧע���������(д������)��
��__________����__________��
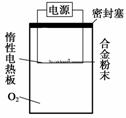
̽����
ʵ�鷽��������x g��þ�Ͻ��ĩ��������ͼ��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������ա�
�������ۣ�
(1)������Mg��������������ʵ���л���ⶨ��������__________________��
(2)���ÿ�������O2����ʵ�飬�Բⶨ����Ƿ���Ӱ�죿________(��ǡ���)��
ʵ����չ
����̽��һ��̽����ʵ�鷽�������������һ��ʵ�鷽�����ⶨ����þ�Ͻ���þ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
±�����ܷ������з�Ӧ��
2CH3CH2Br��2Na�D��CH3CH2CH2CH3��2NaBr�������л�������Ʒ�Ӧ���ϳɻ��������(����)
A��CH3CH2CH2Br B��CH3CHBrCH2Br
C��CH2BrCH2CH2Br D��CH3CHBrCH2CH2Br
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ��ȡ����Ӧ��ֻ���������ַе㲻ͬ�IJ����������(����)
A��(CH3)2CHCH2CH2CH3
B��(CH3CH2)2CHCH3
C��(CH3)2CHCH(CH3)2
D��(CH3)3CCH2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵������ȷ���ǣ� ��
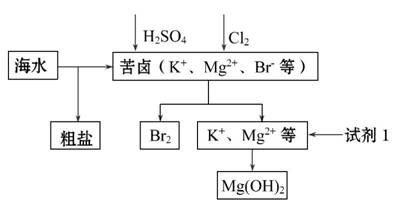
A.����BaCl2��Һ��ȥ�����е�S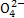
B.�ӿ�±����ȡBr2�ķ�Ӧ�����ӷ���ʽΪ2Br-+Cl2====2Cl-+Br2
C.�Լ�1����ѡ��ʯ����
D.��ҵ�ϣ��������Mg(OH)2ұ������þ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ƣ�NaClO2����һ����Ҫ�ĺ�����������������ˮ�������Լ���֯��Ư�ס��������ⷨ�����������Ƶ�����ͼ����
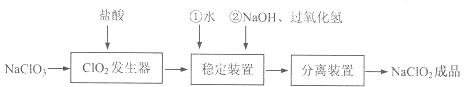
��֪NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2·3H2O��NaClO2�ڼ����������ȶ��Խϸߡ��Իش��������⣺
��1����ClO2��������ͬʱ���������������ڷ������з�����Ӧ�Ļ�ѧ����ʽΪ
��2����NaClO2�ȶ�װ���У�H2O2�� ��ѡ����ţ���
A�������� B����ԭ��
C������������������ԭ�� D���Ȳ���������Ҳ������ԭ��
��3����ʵ����ģ�⡰�������װ�á��еļ�����������е�ʵ������� ����ʵ���˳����д�������ţ���
A������ B������ C����Һ D������ E����ȴ
��4������������֪������pH��2.0ʱ��ClO-2�ܱ�I��ȫ��ԭ��Cl—��
��Һ��Na2S2O3����I2��Ӧ����NaI��Na2S4O6��
���ⶨ��Ʒ��NaClO2�ĺ������ֽ������²�����

�ٲ�����з�����Ӧ�����ӷ���ʽ�� ��
������дﵽ�ζ��յ�ʱ�������� ��
���������ζ���������ȥ��V mL Na2S2O3��Һ������Ʒ��NaClO2���������� ������ĸ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijpH= 1��X��Һ�п��ܺ���Fe2+��A13+��NH4����CO32����SO32����SO42����Cl���е������֣���ȡX��Һ��������ʵ�顣ʵ����̼���������:
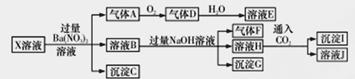
����˵����ȷ����
A.����A��NO2��SO2�Ļ������
B.X�п϶�����Fe2+��NH4����SO42��
C.��ҺE������F���ܷ�����ѧ��Ӧ
D.X�в���ȷ����������SO32����Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������A����(ÿ������ֻ��һ����ԭ��)�����Լ�һ�ֻ���±�أ�����A��ˮ��Ӧ����ȫˮ�������������ԭ�����з�Ӧ�����������ˮ����A���ˮ��Һϡ�ͺ�ֳɼ��ݣ��ֱ����һϵ�е��Լ�����������:
�ټ����������������������ɫ������
�ڼ����Ȼ�����Һ����������;
����Һ���ữ�������������Һ����ɫ��ȥ���ټ������ᱵ��Һ��������ɫ������
(1)�ɴ��ж���ɸû������Ԫ���У����ܴ��ڵ�±����____________��A��ˮ��Ӧ�����ɵ���Һ�к��е����ӿ�����_________________________��
(2)Ҫȷ���û�����ķ���ʽ����ȡ11.90g A����ˮϡ����250.0mL��ȡ25.00mL��Һ���������ĸ��������Һ�����ᱵ��Һ��ʹ������ȫ��������ϴ�ӡ��������أ�Ϊ2.33g����ȷ��A�Ļ�ѧʽ��д��������̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ײ�����ȱλ������(MFe2Ox��3<x<4��M=Mn��Zn��Ni�Ҿ���+2������ͬ)��������(MFe2O4)�����»�ԭ���á������£�����ʹ��ҵ�����е�����������ֽ��ȥ��ת��������ͼ��ʾ��
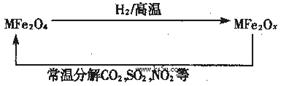
(1)��֪������(MFe2O4)��H2���»�ԭʱ��������Ӧ��MFe2O4��H2�����ʵ���֮��Ϊ 2︰1����ԭ���� MFe2Ox ��x=______��MFe2Ox ��+2������+3��������֮��Ϊ_____��
(2)��������Һ����Fe2O42����ת��ΪFe2��:Fe2O42����2e����8H����2Fe2����4H2O�� KMnO4��Na2CO3��Cu2O��Fe2(SO4)3 ���������е�һ����ʹ������ԭ���̷�����д����������ԭ��Ӧ�����ӷ���ʽ����ƽ_________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com