
 ʯ���ͣ�17��̼ԭ�����ϵ�Һ̬���������ķֽ�ʵ��װ����ͼ��ʾ������������ʡ�ԣ������Թܢ��м���ʯ���ͺ�����������ʯ���ֽ⣩���Թܢڷ�����ˮ�У��Թܢ��м�����ˮ��
ʯ���ͣ�17��̼ԭ�����ϵ�Һ̬���������ķֽ�ʵ��װ����ͼ��ʾ������������ʡ�ԣ������Թܢ��м���ʯ���ͺ�����������ʯ���ֽ⣩���Թܢڷ�����ˮ�У��Թܢ��м�����ˮ��| ���� |
| �� |
| ���� |
| �� |

 ��CH3-CH2=CH2+Br2--��CH3-CH2-CH2
��CH3-CH2=CH2+Br2--��CH3-CH2-CH2 ��CH3-CH2=CH2+Br2--��CH3-CH2-CH2
��CH3-CH2=CH2+Br2--��CH3-CH2-CH2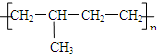 ��
��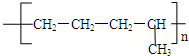 ���ֽṹ��ʽ���ֱ�ΪA��C�ṹ��
���ֽṹ��ʽ���ֱ�ΪA��C�ṹ�� ��CH3-CH2=CH2+Br2--��CH3-CH2-CH2���ӳɷ�Ӧ��
��CH3-CH2=CH2+Br2--��CH3-CH2-CH2���ӳɷ�Ӧ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Cu |
| �� |
| Cu |
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ��ɽһ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
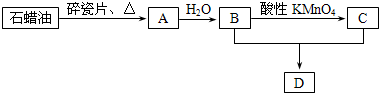
��10�֣�A��һ����Ҫ�Ļ���ԭ�ϣ��ڱ�״���µ��ܶ�Ϊ1.25g/L; C��һ���������ʣ�D�Ǿ��й�����ζ�����������A��B��C��D��һ�������´�������ת����ϵ��ʯ���ͺ�17��̼ԭ�����ϵ�Һ̬���������ַ�Ӧ���������ﱻʡ�ԣ���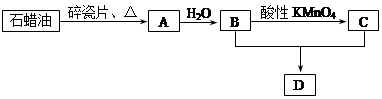
(1)��ҵ�ϣ���ʯ�ͻ�����͡�ú�͡�ʯ���͵ȳɷݵķ����� ��
(2)A��B�Ļ�ѧ����ʽ ����Ӧ������ ��
B��C��D�Ļ�ѧ����ʽ ����Ӧ������ ��
(3)����������������ʯ���ͻ��A�Ĺ����е��м��������һ��ͬ���칹�壬����һ��ȡ����ֻ��һ�֣�������ͬ���칹��Ľṹ��ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ�߶���ѧ�ڵ�һ�ζο����ƻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
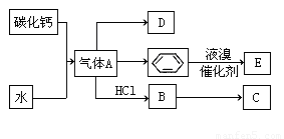
C��һ�ֺϳ���֬�������Ʊ����Ϻͺϳ���ά��D��һ��ֲ���������ڼ����������Դ����ʵ���������»�ѧ��Ӧ��ͼ��գ�
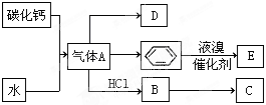
��1��д��A�ĵ���ʽ ��D�����ʽ ��
��2��д��̼������ˮ��Ӧ��ȡA�Ļ�ѧ����ʽ ������Һ�巴Ӧ����E�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��B��C�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
��3��D��������ʯ������ȡ��D��һ�������´�������ת����ϵ��ʯ���ͺ�17��̼ԭ�����ϵ�Һ̬���������ַ�Ӧ���������ﱻʡ�ԣ���G��һ���������ʣ�H�Ǿ��й�����ζ�����������
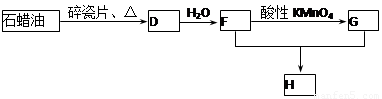
a����ҵ�ϣ���ʯ�ͻ�����͡�ú�͡�ʯ���͵ȳɷݵķ����� ��
b��D��F�Ļ�ѧ����ʽ ���䷴Ӧ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫��ɽ��ɽһ�и߶���ѧ�ڵ�һ�ζο���ѧ�Ծ��������棩 ���ͣ��ƶ���
C��һ�ֺϳ���֬�������Ʊ����Ϻͺϳ���ά��D��һ��ֲ���������ڼ����������Դ����ʵ���������»�ѧ��Ӧ��ͼ��գ�
��1��д��A�ĵ���ʽ ��D�����ʽ ��
��2��д��̼������ˮ��Ӧ��ȡA�Ļ�ѧ����ʽ ������Һ�巴Ӧ����E�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
B��C�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
��3��D��������ʯ������ȡ��D��һ�������´�������ת����ϵ��ʯ���ͺ�17��̼ԭ�����ϵ�Һ̬���������ַ�Ӧ���������ﱻʡ�ԣ���G��һ���������ʣ�H�Ǿ��й�����ζ�����������
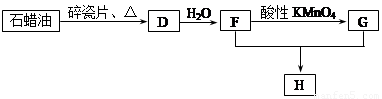
a����ҵ�ϣ���ʯ�ͻ�����͡�ú�͡�ʯ���͵ȳɷݵķ����� ��
b��D��F�Ļ�ѧ����ʽ ���䷴Ӧ������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com