
V”¢W”¢X”¢Y”¢ZŹĒÓÉÖÜĘŚ±ķÖŠ1”«20ŗŲæ·ÖŌŖĖŲ×é³ÉµÄ5ÖÖ»ÆŗĻĪļ£¬ĘäÖŠV”¢W”¢X”¢Z¾łĪŖĮ½ÖÖŌŖĖŲ×é³É”£ÉĻŹö5ÖÖ»ÆŗĻĪļÉę¼°µÄĖłÓŠŌŖĖŲµÄŌ×ÓŠņŹżÖ®ŗĶµČÓŚ35”£ĖüĆĒÖ®¼äµÄ·“Ó¦¹ŲĻµČēĻĀĶ¼£ŗ

£Ø1£©5ÖÖ»ÆŗĻĪļ·Ö±šŹĒV________”¢W________”¢X________”¢Y________”¢Z________£»(Ģī»ÆѧŹ½)
£Ø2£©ÓÉÉĻŹö5ÖÖ»ÆŗĻĪļÖŠµÄij2ÖÖ»ÆŗĻĪļ·“Ó¦æÉÉś³ÉŅ»ÖÖŠĀ»ÆŗĻĪļ£¬Ėü°üŗ¬ĮĖ5ÖÖ»ÆŗĻĪļÖŠµÄĖłÓŠŌŖĖŲ£¬Éś³ÉøĆ»ÆŗĻĪļµÄ»Æѧ·½³ĢŹ½ŹĒ____________________________£»
£Ø3£©VµÄµē×ÓŹ½ŹĒ________________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄæĒ°£¬Ęū³µĪ²ĘųŅŃ³ÉĪŖŠķ¶ą“ó³ĒŹŠæÕĘųµÄÖ÷ŅŖĪŪČ¾”£Ęū³µĪ²ĘųÖŠŗ¬ÓŠCO”¢NOµČ¶ąÖÖĪŪČ¾Īļ”£
£Ø1£©Ęū³µČ¼ĮĻÖŠŅ»°ć²»ŗ¬µŖ£¬Ī²ĘųÖŠĖłŗ¬µÄNO²śÉśµÄŌŅņŹĒ £¬ »Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ÖĪĄķĘū³µĪ²ĘųÖŠNOŗĶCOµÄŅ»ÖÖ·½·ØŹĒ£ŗŌŚĘū³µµÄÅÅĘų¹ÜÉĻ×°ÉĻŅ»øö“ß»Æ×Ŗ»Æ×°ÖĆ£¬Ź¹NOŗĶCO·“Ó¦£¬Éś³ÉCO2ŗĶ N2”£·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©ĻĀĮŠ“ėŹ©ÖŠ£¬ÄܼõÉŁ»ņæŲÖĘĘū³µĪ²ĘųĪŪČ¾ÓŠŠ§ĒŅæÉŠŠµÄŹĒ ”£
¢Ł ÖʶØŃĻøńµÄĪ²ĘųÅŷűź×¼£¬²¢ŃĻøńÖ“ŠŠ”£ ¢Ś æŖ·¢Ēå½ąÄÜŌ“£¬ČēĒāÄÜĘū³µ”¢Ģ«ŃōÄÜĘū³µµČ”£ ¢Ū ŹŠĆń³öŠŠ“ų·Ą¶¾Ćę¾ß”£ ¢Ü ŹŠĆń“óĮæŅĘ¾Ó³ĒŹŠ½¼Ēų”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¼×”¢ŅŅ”¢±ūČżÖÖĪļÖŹÖ®¼äÓŠČēĻĀ×Ŗ»Æ¹ŲĻµ£ŗ
¼×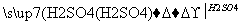 ŅŅ
ŅŅ ±ū
±ū ¼×
¼×
£Ø1£©Čō¼×ŗĶ±ū¶¼ŹĒ²»ČÜÓŚĖ®µÄ°×É«¹ĢĢåĪļÖŹ£¬¼ČÄÜČÜÓŚŃĪĖįÓÖÄÜČÜÓŚĒāŃõ»ÆÄĘČÜŅŗ”£
Ōņ¼×ŹĒ________£¬±ūŹĒ________(Ģī»ÆѧŹ½)”£Š“³ö”°ŅŅ ±ū”±×Ŗ»ÆµÄĄė×Ó·½³ĢŹ½£ŗ______________________________”£
±ū”±×Ŗ»ÆµÄĄė×Ó·½³ĢŹ½£ŗ______________________________”£
£Ø2£©ČōŅŅČÜŅŗÖŠ¼ÓČėKSCNČÜŅŗ£¬ÓŠŗģÉ«³öĻÖ£¬Ōņ¼×ŹĒ______£¬±ūŹĒ________(Ģī»ÆѧŹ½)”£Š“³ö”°¼× ŅŅ”±×Ŗ»ÆµÄĄė×Ó·½³ĢŹ½£ŗ____________________________”£
ŅŅ”±×Ŗ»ÆµÄĄė×Ó·½³ĢŹ½£ŗ____________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŠÅĻ¢Ź±“ś²śÉśµÄ“óĮæµē×ÓĄ¬»ų¶Ō»·¾³¹¹³ÉĮĖ¼«“óµÄĶžŠ²”£Ä³”°±ä·ĻĪŖ±¦”±Ń§ÉśĢ½¾æŠ”×齫Ņ»Åś·ĻĘśµÄĻßĀ·°å¼ņµ„“¦Ąķŗó£¬µĆµ½ŗ¬70% Cu”¢25% Al”¢4% Fe¼°ÉŁĮæAu”¢PtµČ½šŹōµÄ»ģŗĻĪļ£¬²¢Éč¼Ę³öČēĻĀÖʱøĮņĖįĶŗĶ ĮņĖįĀĮ¾§ĢåµÄĀ·Ļߣŗ
ĮņĖįĀĮ¾§ĢåµÄĀ·Ļߣŗ
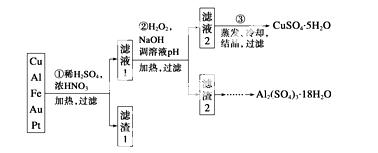
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©µŚ¢Ł²½CuÓėĖį·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________________£»µĆµ½ĀĖŌü1µÄÖ÷ŅŖ³É·ÖĪŖ____________”£
£Ø2£©µŚ¢Ś²½¼ÓH2O2µÄ×÷ÓĆŹĒ______________£¬Ź¹ÓĆH2O2µÄÓŵćŹĒ______________£»µ÷ČÜŅŗpHµÄÄæµÄŹĒŹ¹______________Éś³É³Įµķ”£
£Ø3£©ÓƵŚ¢Ū²½ĖłµĆCuSO4·5H2OÖʱøĪŽĖ®CuSO4µÄ·½·ØŹĒ_______________________”£
£Ø4£©ÓÉĀĖ Ōü2ÖĘČ”Al2(SO4)3·18H2O £¬Ģ½¾æŠ”×éÉč¼ĘĮĖČżÖÖ·½°ø£ŗ
Ōü2ÖĘČ”Al2(SO4)3·18H2O £¬Ģ½¾æŠ”×éÉč¼ĘĮĖČżÖÖ·½°ø£ŗ

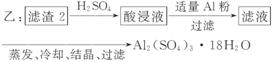
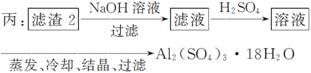
ÉĻŹöČżÖÖ·½°øÖŠ£¬________·½°ø²»æÉŠŠ£¬ŌŅņŹĒ____________________________
_____________________________________________________________________£»
“ÓŌ×ÓĄūÓĆĀŹ½Ē¶Čæ¼ĀĒ£¬__________·½°øøüŗĻĄķ”£
£Ø5£©Ģ½¾æŠ”×éÓƵĪ¶Ø·Ø²ā¶ØCuSO4·5H 2O (Mr£½250)ŗ¬Įæ”£Č”a gŹŌŃłÅä³É100 mLČÜŅŗ£¬Ćæ“ĪČ”20.00 mL£¬Ļū³żøÉČÅĄė×Óŗó£¬ÓĆc mol·L£1 EDTA(H2Y2£)±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć£¬Ę½¾łĻūŗÄEDTAČÜŅŗb mL”£µĪ¶Ø·“Ó¦ČēĻĀ£ŗCu2£«£«H2Y2£===CuY2££«2H£«
2O (Mr£½250)ŗ¬Įæ”£Č”a gŹŌŃłÅä³É100 mLČÜŅŗ£¬Ćæ“ĪČ”20.00 mL£¬Ļū³żøÉČÅĄė×Óŗó£¬ÓĆc mol·L£1 EDTA(H2Y2£)±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć£¬Ę½¾łĻūŗÄEDTAČÜŅŗb mL”£µĪ¶Ø·“Ó¦ČēĻĀ£ŗCu2£«£«H2Y2£===CuY2££«2H£«
Š“³ö¼ĘĖćCuSO4·5H2OÖŹĮæ·ÖŹżµÄ±ķ“ļŹ½w£½______________________________£»
ĻĀĮŠ²Ł×÷»įµ¼ÖĀCuSO4·5H2Oŗ¬ĮæµÄ²ā¶Ø½į¹ūĘ«øߵďĒ________”£
a£®Ī“øÉŌļ׶ŠĪĘæ
b£®µĪ¶ØÖÕµćŹ±µĪ¶Ø¹Ü¼ā×ģÖŠ²śÉśĘųÅŻ
c£®Ī“³ż¾»æÉÓėEDTA·“Ó¦µÄøÉČÅĄė×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
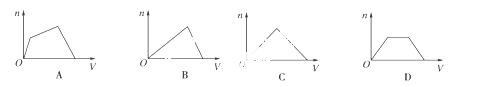
½«×ćĮæCO2ĶØČėKOHŗĶCaOH2 µÄ»ģŗĻĻ”ČÜŅŗÖŠ£¬Éś³É³ĮµķµÄĪļÖŹµÄĮænŗĶĶØČėCO2µÄĢå»ż£ØV£©µÄ¹ŲĻµÕżČ·µÄŹĒ£Ø £©
µÄ»ģŗĻĻ”ČÜŅŗÖŠ£¬Éś³É³ĮµķµÄĪļÖŹµÄĮænŗĶĶØČėCO2µÄĢå»ż£ØV£©µÄ¹ŲĻµÕżČ·µÄŹĒ£Ø £©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ (””””)
A£®ŅņĪŖSO2¾ßÓŠĘư׊Ō£¬ĖłŅŌĖüÄÜŹ¹Ę·ŗģČÜŅŗ”¢äåĖ®”¢ĖįŠŌøßĆĢĖį¼ŲČÜŅŗ”¢ŹÆČļŹŌŅŗĶŹÉ«
B£®ÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄ²»Ņ»¶ØŹĒSO2
C£®SO2”¢ĘÆ°×·Ū”¢»īŠŌĢ攢Na2O2¶¼ÄÜŹ¹ŗģÄ«Ė®ĶŹÉ«£¬ĘäŌĄķĻąĶ¬
D£®SO2ŗĶCl2µČĪļÖŹµÄ Įæ»ģŗĻŗóĶØČė×°ÓŠŹŖČóµÄÓŠÉ«²¼ĢõµÄ¼ÆĘųĘæÖŠ£¬Ęư׊§¹ūøüŗĆ
Įæ»ģŗĻŗóĶØČė×°ÓŠŹŖČóµÄÓŠÉ«²¼ĢõµÄ¼ÆĘųĘæÖŠ£¬Ęư׊§¹ūøüŗĆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
A£®½«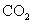 ĶØČė
ĶØČė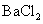 ČÜŅŗÖŠÖĮ±„ŗĶ£¬ĪŽ³Įµķ²śÉś£»ŌŁĶØČė
ČÜŅŗÖŠÖĮ±„ŗĶ£¬ĪŽ³Įµķ²śÉś£»ŌŁĶØČė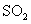 £¬²śÉś³Įµķ
£¬²śÉś³Įµķ
B£®ŌŚĻ”ĮņĖįÖŠ¼ÓČėĶ·Ū£¬Ķ·Ū²»Čܽā£»ŌŁ¼ÓČė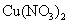 ¹ĢĢ壬Ķ·ŪČŌ²»Čܽā
¹ĢĢ壬Ķ·ŪČŌ²»Čܽā
C£®Ļņ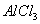 ČÜŅŗÖŠµĪ¼Ó°±Ė®£¬²śÉś°×É«³Įµķ£»ŌŁ¼ÓČė¹żĮæ
ČÜŅŗÖŠµĪ¼Ó°±Ė®£¬²śÉś°×É«³Įµķ£»ŌŁ¼ÓČė¹żĮæ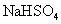 ČÜŅŗ£¬³ĮµķĻūŹ§
ČÜŅŗ£¬³ĮµķĻūŹ§
D£®“æŠæÓėĻ”ĮņĖį·“Ó¦²śÉśĒāĘųµÄĖŁĀŹ½ĻĀż£»ŌŁ¼ÓČėÉŁĮæ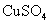 ¹ĢĢ壬ĖŁĀŹ²»øıä
¹ĢĢ壬ĖŁĀŹ²»øıä
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°Ń0.05 mol NaOH¹ĢĢå·Ö±š¼ÓČėµ½100 mLĻĀĮŠŅŗĢåÖŠ£¬ČÜŅŗµÄµ¼µēÄÜĮ¦±ä»Æ×īŠ”µÄŹĒ(””””)
A£®×ŌĄ“Ė®
B£®0.5 mol”¤L£1ŃĪĖį
C£®0.5 mol”¤L£1 CH3COOHČÜŅŗ
D£®0.5 mol”¤L£1 KClČÜŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A£®ŌŚŅ»¶ØĪĀ¶ČĻĀAgClĖ®ČÜŅŗÖŠ£¬Ag£«ŗĶCl£ÅØ¶ČµÄ³Ė»żŹĒŅ»øö³£Źż
B£®AgClµÄKsp£½1.8”Į10£10 mol2”¤L£2£¬ŌŚČĪŗĪŗ¬AgCl¹ĢĢåµÄČÜŅŗÖŠ£¬[Ag£«]£½[Cl£]ĒŅAg£«ÓėCl£ÅØ¶ČµÄ³Ė»żµČÓŚ1.8”Į10£10 mol2”¤L£2
C£®ĪĀ¶ČŅ»¶ØŹ±£¬µ±ČÜŅŗÖŠAg£«ŗĶCl£ÅØ¶ČµÄ³Ė»żµČÓŚKspÖµŹ±£¬“ĖČÜŅŗĪŖAgClµÄ±„ŗĶČÜŅŗ
D£®Ļņ±„ŗĶAgClĖ®ČÜŅŗÖŠ¼ÓČėŃĪĖį£¬KspÖµ±ä“ó
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com