
| A�� | �����½�pH=12�İ�ˮ��ˮϡ�ͣ�ϡ��������Һ��$\frac{c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$��С | |
| B�� | ͬŨ�ȵĴ����ƺʹ����������Һ�У�c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | 25����ͬpH�Ģ�CH3COONa��NaHCO3��NaAlO2������Һ�е�c��Na+�����٣��ڣ��� | |
| D�� | Ũ�Ⱦ�Ϊ0.1mol•L-1��KHS��HCl��������Һ�У�c��HS-��+c��H2S��=0.1mol•L-1 |
���� A����ˮ��ˮϡ�ͣ��ٽ�һˮ�ϰ��ĵ��룬��n��OH-������n��NH3��H2O����С��
B��ͬŨ�ȵĴ����ƺʹ����������Һ����ĵ�������ε�ˮ�⣬�����ԣ�
C�������Դ��̼�������������֪��ͬpH�Ģ�CH3COONa��NaHCO3��NaAlO2������Һ��Ũ��Ϊ�٣��ڣ��ۣ�
D����������Һ����Һ���Ϊԭ����2������������غ������
��� �⣺A����ˮ��ˮϡ�ͣ��ٽ�һˮ�ϰ��ĵ��룬��n��OH-������n��NH3��H2O����С����ϡ��������Һ��$\frac{c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$����A����
B��ͬŨ�ȵĴ����ƺʹ����������Һ����ĵ�������ε�ˮ�⣬�����ԣ�������Ũ��Ϊc��CH3COO-����c��Na+����c��H+����c��OH-��������ѭ����غ㣬��B����
C�������Դ��̼�������������֪��ͬpH�Ģ�CH3COONa��NaHCO3��NaAlO2������Һ��Ũ��Ϊ�٣��ڣ��ۣ���������Һ�е�c��Na+�����٣��ڣ��ۣ���C��ȷ��
D����������Һ����Һ���Ϊԭ����2�����������غ��֪c��S2-��+c��HS-��+c��H2S��=$\frac{0.1mol/L}{2}$=0.05mol•L-1����D����
��ѡC��
���� ���⿼��������ʵĵ��뼰����Ũ�ȵıȽϣ�Ϊ��Ƶ���㣬���յ��롢ˮ�⼰����غ㡢�����غ�Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �մ�--NaHCO3 | B�� | ����--C2H5OH | C�� | ����-KAl��SO4��2 | D�� | ��ʯ��-CaO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
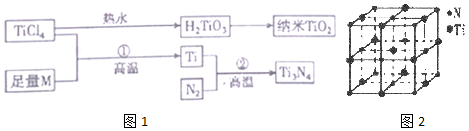
| I1 | I2 | I3 | I4 | I5 | |
| ������/��kJ•mol-1�� | 738 | 1451 | 7733 | 10 540 | 13 630 |
| ���Ӿ��� | NaCl | KC1 | CaO |
| ������/��kJ•mol-1�� | 786 | 715 | 3401 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���³�ѹ�£�46 gN2O4�к���ԭ����Ϊ2NA | |
| B�� | ���³�ѹ�£�11.2 LNH3�к����ۼ�����ĿΪ1.5NA | |
| C�� | ��⾫��ͭ������������32 gʱ��·��ת�Ƶ�������ΪNA | |
| D�� | 1 L 0.2 mol/LNa2SO4��Һ�к���������������Ϊ0.6NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ�������£���1molN2��3molH2��Ϸ�����Ӧ��ת�Ƶĵ�������Ϊ6NA | |
| B�� | 1L0.1mol•L-1��Na2CO3��Һ�������ӵ���������0.1NA | |
| C�� | ��FeI2��Һ��ͨ������Cl2������2molFe2+������ʱ������Cl2�ķ�����ΪNA | |
| D�� | 1mol-CH3�������ĵ�������Ϊ10NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
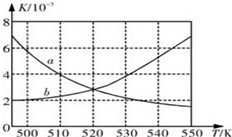 ��Ϊ�˼���CO���ŷţ�ij�����о�С����CO��H2 Ϊԭ�Ϻϳ������Դ�����ѣ�DME������Ӧ���£�4H2��g��+2CO��g��
��Ϊ�˼���CO���ŷţ�ij�����о�С����CO��H2 Ϊԭ�Ϻϳ������Դ�����ѣ�DME������Ӧ���£�4H2��g��+2CO��g��| ʱ��/min | 0 | 20 | 40 | 80 | 100 |
| n��H2��/mol | 2.0 | 1.4 | 0.85 | 0.4 | - |
| n��CO��/mol | 1.0 | - | 0.425 | 0.2 | 0.2 |
| n��CH3OCH3��/mol | 0 | 0.15 | - | - | 0.4 |
| n��H2O��/mol | 0 | 0.15 | 0.2875 | 0.4 | 0.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
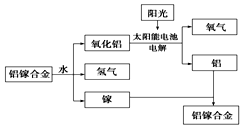 �����նȴ�ѧ�о�������һ���������غϽ��Ʊ��������¹��գ���ͼ��ʾ���������йظù��յ�˵��������ǣ�������
�����նȴ�ѧ�о�������һ���������غϽ��Ʊ��������¹��գ���ͼ��ʾ���������йظù��յ�˵��������ǣ�������| A�� | ���غϽ���ˮ��Ӧ�Ļ�ѧ����ʽΪ2Al+3H2O$\frac{\underline{\;һ������\;}}{\;}$Al2O3+3H2�� | |
| B�� | �ܷ�ӦʽΪ2H2O$\frac{\underline{\;һ������\;}}{\;}$2H2��+O2�� | |
| C�� | �ù����У�������ת����ʽֻ������ | |
| D�� | ���غϽ����ѭ��ʹ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2Al2O3+3C$\frac{\underline{\;����\;}}{\;}$4Al+3CO2�� | B�� | CuCl2$\frac{\underline{\;���\;}}{\;}$Cu+Cl2�� | ||
| C�� | Fe3O4+4CO$\frac{\underline{\;����\;}}{\;}$3Fe+4CO2 | D�� | 2HgO$\frac{\underline{\;���\;}}{\;}$2Hg+O2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com