
��1������Һ��ʯ��������Ҫ�ɷ�֮һ�Ƕ���(C4H10)����10 kg������ȫȼ�ղ����ɶ�����̼�����Һ̬ˮʱ���ų�������Ϊ5��105 kJ����д������ȼ�յ��Ȼ�ѧ����ʽ�� ����֪1molҺ̬ˮ����ʱ��Ҫ����44 kJ��������ӦC4H10(g)+6.5O2(g)=4CO2(g)+5H2O(g)�Ħ�H= ��
��2����ͬѧ�ö��������Ϊԭ������һȼ�յ�أ�ͨ�붡���һ��Ϊ ��������ϡ����Ϊ�������Һʱ����������ӦʽΪ ��
��3����֪:Fe(s) +1/2O2(g)=FeO(s) ��H=��272.0kJ��mol-1
2Al(s)+3/2O2(g)=Al2O3(s) ��H=��1675.7kJ��mol-1
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ�� ��
��4����֪��1 mol H��H����1 molN��H����1 molN��N���ֱ���Ҫ��������akJ��bkJ��ckJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ ��

���������������1�����������������ʵ�������ϻ�ѧ����ʽ��Ӧ�����ʵ������㷴Ӧ�ų��������������Ȼ�ѧ����ʽ��д��������ע���ʾۼ�״̬�ͷ�Ӧ�ʱ�д����������֪H2O(l)=H2O(g) ��H=44 kJ��mol-1,���ø�˹���ɼ���ɵã�
��2������--����ȼ�ϵ�ع���ʱ������ʧ���ӷ���������Ӧ����������ϡ����Ϊ�������Һʱ����ϵ缫��Ӧʽ����д���ɿɵã�
��3����������Ȼ�ѧ����ʽ��ϸ�˹����д�����Ȼ�ѧ��Ӧ����ʽ��������ʽ��-�١�3��2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ?mol-1��
(4)���ݡ�H=��Ӧ��ļ���֮�͡�������ļ���֮�Ϳ���ĺϳɰ���Ӧ���ʱ�Ϊ(3a+b-6c) kJ��mol-1�������Ȼ�ѧ����ʽ��д��������ע���ʾۼ�״̬�ͷ�Ӧ�ʱ�д����
���㣺�����Ȼ�ѧ����ʽ����д���ʱ�ļ����ȼ�ϵ�صȡ�


 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һ�������·�����Ӧ��CO(g)��2H2(g) CH3OH(g)����ҵ��������CO����ȼ�ϼ״���
CH3OH(g)����ҵ��������CO����ȼ�ϼ״���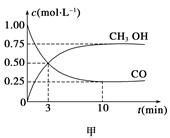
��1����ͼ�Ƿ�ӦʱCO��CH3OH(g)��Ũ����ʱ��仯������ӷ�Ӧ��ʼ��ƽ�⣬��H2Ũ�ȱ仯��ʾƽ����Ӧ����v(CO)��__________________��
��2����ͼ��ʾ�÷�Ӧ���й����������ı仯������b�µķ�Ӧ����Ϊ ���÷�Ӧ���ʱ���________(���H<0����H>0��)��д����Ӧ���Ȼ�ѧ����ʽ��____________________________________��ѡ�����˵Ĵ���__________(��ܡ����ܡ�)�ı�÷�Ӧ�ķ�Ӧ�ȡ�
��3���÷�Ӧƽ�ⳣ��K�ı���ʽΪ_____________________________________________��
�¶����ߣ�ƽ�ⳣ��K________(����������䡱��С��)��
��4�����������£����д�ʩ����ʹ �������____________��
�������____________��
a�������¶�
b������He��
c���ٳ���1 mol CO��2 mol H2
d��ʹ�ô���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ͼ�ӡˢ��·��Ļ������ÿ�ʵ����Դ��������������Ⱦ���Ͼ�ӡˢ��·�徭������룬�ܵõ��ǽ�����ĩ�ͽ�����ĩ��
��1�����д���ӡˢ��·��ǽ�����ĩ�ķ����У������ϻ�������������� ������ĸ����
| A�����ѽ��γ�ȼ�� | B��¶����� | C����Ϊ�л����Ͻ������ϵ�ԭ�� | D��ֱ������ |

| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ͭƽ���ܽ����ʣ���10-3 mol·L-1·min-1�� | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
 H
H
 H��
H�� H= ��
H= ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2SO2(g)��O2(g) 2SO3(g)����Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 mol SO3(g)�Ħ�H����99 kJ/mol��
2SO3(g)����Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 mol SO3(g)�Ħ�H����99 kJ/mol��
��ش��������⣺
��1��ͼ��A��C�ֱ��ʾ__________��__________��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿____________���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ�___________��
��2��ͼ�Ц�H��__________kJ/mol��
��3�������Ӧ����v(SO2)Ϊ0.05 mol/(L��min)����v(O2)��____mol/(L��min)
��4����֪�������ȼ����Ϊ296 kJ/mol��������S(s)����3 mol SO3(g)�Ħ�H��_ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
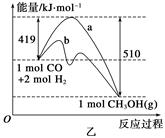
��˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g)��H���D24��8kJ?mol-1
��3Fe2O3(s)+CO(g)=2Fe3O4(s)+CO2(g)��H���D47��2kJ?mol-1
��Fe3O4(s)+CO(g)=3FeO(s)+CO2(g)��H��+640��5kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��__________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ྻú�������о����������൱�ձ飬������Աͨ�������ú������¯�н�����������ˮ�����ķ�����������������ֵ�ߴ�122500~16000 kJ��m-3��ú̿��������Ҫ�ɷ���CO��H2��CO��H2����Ϊ��Դ�ͻ���ԭ�ϣ�Ӧ��ʮ�ֹ㷺��
��1����֪��C(s)+O2(g)=CO2(g) ��H1����393.5 kJ��mol-1 ��
2H2(g)+O2(g)=2H2O(g) ��H2����483.6 kJ��mol-1 ��
C(s)+H2O(g)=CO(g)+H2(g) ��H3����131.3 kJ��mol-1 ��
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= kJ��mol-1����״���µ�ú̿����CO��H2��33.6 L��������ȫ��Ӧ����CO2��H2O����Ӧ������ת�� mol e-��
��2���ܱ������г���10 mol CO��20 mol H2���ڴ��������·�Ӧ���ɼ״���CO(g)+2H2(g) 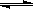 CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��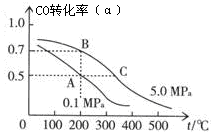
����A��B�����ʾ��ijʱ�̴ﵽ��ƽ��״̬����ʱ��A��ʱ���������ΪVAL������¶��µ�ƽ�ⳣ��K= ��A��B����ʱ���������ʵ����ʵ���֮��Ϊn(A)����n(B)��= ��
����A��C���㶼��ʾ�ﵽ��ƽ��״̬�����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��tA tC������ڡ�����С�ڡ����ڡ�����
���ڲ��ı䷴Ӧ������������£�Ϊ���CO��ת���ʿɲ�ȡ�Ĵ�ʩ�� ��
A.���� B.��ѹ C.ʹ�ô��� D.���״��ӻ����ϵ�з������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2011��11��1�գ��ҹ��������Ƶġ���������F��ң�����ػ���������۰˺š��ɴ�����̫��Ԥ�������������롰�칬һ�š�Ŀ�������ʵ�ֳɹ��Խӡ�ƫ�����£�C2H8N2)������������(N2O4)�dz���ϵ�л���ij����ƽ�������ش�����������⣺
��1��ƫ�����£�C2H8N2)������������(N2O4)��Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ��
C2H8N2��2N2O4 3N2��2X��4H2O
3N2��2X��4H2O
��X�Ļ�ѧʽΪ____________��ѡ������ѡ��ı����ĸ����
A��O2 B��CO2 C��H2
��2��1 gҺ̬ƫ��������������Һ̬������������ȫ��Ӧ������̬����ų�Q kJ����������ͬ������0.1 molƫ�����·����÷�Ӧ�ܷų�������Ϊ_____________kJ (ѡ�����б����ĸ)��
A��6Q B��30Q C��60Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ�˼���CO�Դ�������Ⱦ��ij�о���ѧϰС�����о�CO��H2O��Ӧת��Ϊ��ɫ��ԴH2����֪��
2CO(g)+O2(g)��2CO2(g) ��H����566kJ��moL��1
2H2(g)+O2(g)��2H2O(g) ��H����483.6KJ��moL��1
H2O (g)��H2O(l) ��H����44.0KJ��moL��1
��1�������ı�ȼ���ȡ�H�� kJ��moL��1
��2��д��CO��H2O(g)��������CO2��H2���Ȼ�ѧ����ʽ
��3���� 1L�������������м���1.00mol CO��1.00mol H2O(g)����t��ʱ��Ӧ���ﵽƽ�⣬���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��1����t��ʱCO��ת����Ϊ ����Ӧ�ﵽƽ��������¶ȣ���ʱƽ�ⳣ���� �����������䡱��С������ƽ�⽫�� ����������桱�������ƶ���
��4����CO��H2O��Ӧת��Ϊ��ɫ��ԴH2�У�Ϊ�����CO��ת���ʣ��ɲ�ȡ�Ĵ�ʩ�� ��
| A�������COŨ�� | B�������H2O(g)Ũ�� | C��ʹ�ô��� | D�������¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о� ��
��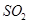 ��CO�ȴ�����Ⱦ����IJ���������������Ҫ���塣
��CO�ȴ�����Ⱦ����IJ���������������Ҫ���塣
��1�� ��ʹ
��ʹ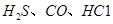 �������������ڶ����ⶨCO�ĺ�������֪��
�������������ڶ����ⶨCO�ĺ�������֪��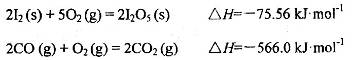
д��CO��g����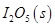 ��Ӧ����
��Ӧ����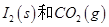 ���Ȼ�ѧ����ʽ��________________��
���Ȼ�ѧ����ʽ��________________��
��2��CO������ȼ�ϵ�أ���KOH��Һ������ʣ��������ֱ����CO�Ϳ��������������У�K+����_______��(���������)��������Ӧ����ʽΪ��___________________��
��3�����Ͱ��������������Ļ�ѧԭ���Dz��ð�ˮ���������е�SO2������һ��������
�����������ղ��ﷴӦ���ü������ŵ�����ܻ�������SO2�⣬���ܵõ�һ�ָ��Ϸ��ϡ�
�ٸø��Ϸ��Ͽ��ܵĻ�ѧʽΪ___________(д��һ�ּ���)��
������ˮ��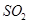 ǡ����ȫ��Ӧ�������Σ����ʱ��Һ��________��(��ᡱ�)��
ǡ����ȫ��Ӧ�������Σ����ʱ��Һ��________��(��ᡱ�)��
������������ʵĵ���ƽ�ⳣ�����£���ˮ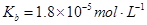

���������Һ��ͨ��________�����ʹ��Һ�����ԡ�(�SO2����NH3��)
��ʱ��Һ�� ________2�������������������
________2�������������������
��4��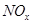 ����ǿ����Һ�����������Ρ������������£�FeSO4��Һ�ܽ�
����ǿ����Һ�����������Ρ������������£�FeSO4��Һ�ܽ�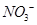 ��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ___________________________________��
��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ___________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com