
·ÖĪö ÉčøĆĘųĢ¬ĢžµÄ×é³ÉĪŖCxHy£¬Č¼ÉÕ·½³ĢŹ½ĪŖCxHy+£Øx+$\frac{y}{4}$£©O2$\stackrel{µćČ¼}{”ś}$xCO2+$\frac{y}{2}$H2O£¬NaOHČÜŅŗĪüŹÕ40mLĪŖČ¼ÉÕÉś³ÉCO2µÄĢå»ż£¬²śĪļĶعżÅØĮņĖį£¬ŌŁ»Öø“ÖĮŹŅĪĀ£¬ĘųĢåĢå»ż¼õÉŁĮĖ50mL£¬øł¾Ż·½³ĢŹ½ĄūÓĆ²īĮæ·Ø¼ĘĖć£®
½ā“š ½ā£ŗÉčøĆĘųĢ¬ĢžµÄ×é³ÉĪŖCxHy£¬
ŌņCxHy+£Øx+$\frac{y}{4}$£©O2$\stackrel{µćČ¼}{”ś}$xCO2+$\frac{y}{2}$H2O”÷V
1 x+$\frac{y}{4}$ x 1+$\frac{y}{4}$
20 40 50
$\frac{1}{20}=\frac{x}{40}$£¬½āµĆx=2£¬
$\frac{1}{20}=\frac{1+\frac{y}{4}}{50}$£¬½āµĆy=6£¬
£Ø1£©ÓÉÉĻŹö·ÖĪöæÉÖŖ£¬øĆĘųĢ¬ĢžµÄ·Ö×ÓŹ½ĪŖC2H6£¬¹Ź“š°øĪŖ£ŗC2H6£»
£Ø2£©øĆĘųĢ¬Ģž·¢ÉśŅ»ĀČČ”“ś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖCH3CH3+Cl2 $\stackrel{¹āÕÕ}{”ś}$CH3CH2Cl+HCl£¬
¹Ź“š°øĪŖ£ŗCH3CH3+Cl2 $\stackrel{¹āÕÕ}{”ś}$CH3CH2Cl+HCl£»
£Ø3£©øĆĘųĢ¬ĢžµÄ¶žĀČČ”“śĪļÓŠClCH2CH2Cl”¢CH3CHCl2£¬¹²2ÖÖ£¬¹Ź“š°øĪŖ£ŗ2£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļ·Ö×ÓŹ½¼ĘĖćµÄČ·¶Ø£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕĢžµÄČ¼ÉÕ¹ęĀÉ”¢Ō×ÓŹŲŗć”¢²īĮæ·Ø¼ĘĖćĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·ÖĪöÓė¼ĘĖćÄÜĮ¦µÄ漲飬עŅā²īĮæ·ØµÄÓ¦ÓĆ£¬ĢāÄæÄŃ¶Č²»“ó£®


 ĒįĖɶį¹ŚČ«ÄÜÕĘæŲ¾ķĻµĮŠ“š°ø
ĒįĖɶį¹ŚČ«ÄÜÕĘæŲ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Čt2/”ę | ĪĀ¶Č²ī £Øt2-t1£©/”ę | |||
| HCl | NaOH | Ę½¾łÖµ | |||
| 1 | 25.5 | 25.0 | 25.25 | 28.5 | 3.25 |
| 2 | 24.5 | 24.2 | 24.45 | 27.6 | 3.15 |
| 3 | 25.0 | 24.5 | 24.75 | 26.5 | 1.75 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆČóŹŖµÄpHŹŌÖ½²ā¶ØĻ”¼īČÜŅŗµÄpH£¬²ā¶ØֵʫŠ” | |
| B£® | ÓĆ¼īŹ½µĪ¶Ø¹ÜĮæČ”22.00mLµÄKMnO4ČÜŅŗ | |
| C£® | ŌŚĪ“ÖŖŅŗÖŠµĪ¼ÓBaCl2ČÜŅŗ²śÉś°×É«³Įµķ£¬¼ÓĻ”ĻõĖį£¬³Įµķ²»Čܽā£¬ĖµĆ÷øĆĪ“ÖŖŅŗÖŠŅ»¶Øŗ¬ÓŠSO42-»ņSO32- | |
| D£® | ŹµŃéŹŅÖʱøĒāŃõ»ÆĢś½ŗĢåµÄ·½·ØŹĒ½«ĒāŃõ»ÆÄĘĻ”ČÜŅŗµĪČė±„ŗĶĀČ»ÆĢśČÜŅŗÖŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ŗĶéÓŠ4ÖÖĶ¬·ÖŅģ¹¹Ģ壬ĖüĆĒµÄČŪ·Šµć²»ĻąĶ¬ | |
| B£® | ŌŚŅ»¶ØĢõ¼žĻĀ£¬±½ÓėŅŗä唢ĻõĖį×÷ÓĆÉś³Éäå±½”¢Ļõ»ł±½µÄ·“Ó¦¶¼ŹōÓŚČ”“ś·“Ó¦ | |
| C£® | ĶéĢžµÄĶØŹ½ĪŖCnH2n+2£¬ĖęnÖµŌö“ó£¬Ģ¼ŌŖĖŲµÄÖŹĮæ°Ł·Öŗ¬ĮæÖš½„Ōö“ó | |
| D£® | ¾ŪŗĻĪļ Óɵ„ĢåCH3CH=CH2ŗĶCH2=CH2¼Ó¾ŪÖĘµĆ Óɵ„ĢåCH3CH=CH2ŗĶCH2=CH2¼Ó¾ŪÖĘµĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĢśÖĘĘ·øÆŹ“Ź±¼ČæÉ×÷Õż¼«Ņ²æÉ×÷øŗ¼« | |
| B£® | ĢśÖĘĘ·øÆŹ“Ź±Ģśµē¼«·¢Éś·“Ó¦ĪŖ£ŗFe-3e-=Fe2+ | |
| C£® | ĢśÖĘĘ·øÆŹ“Ź±»ņ·¢ÉśĪöĒāøÆŹ“»ņ·¢ÉśĪüŃõøÆŹ“ | |
| D£® | ĢśÖĘĘ·Į¬½ÓµēŌ“Õż¼«æÉ·ĄÖ¹øÆŹ“ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ÓČė¹żĮæµÄBa£ØOH£©2ČÜŅŗŹ±£ŗ2Al3++3SO42-+3Ba2++6OH-ØT2Al£ØOH£©3”ż+3BaSO4”ż | |
| B£® | ČÜŅŗĒ”ŗĆ³ŹÖŠŠŌŹ±£ŗ2Al3++3SO42-+3Ba2++6OH-ØT2Al£ØOH£©3”ż+3BaSO4”ż | |
| C£® | SO42-Ąė×ÓĒ”ŗĆĶźČ«³ĮµķŹ±£ŗAl3++SO42-+Ba2++4OH-ØTAlO2-+BaSO4”ż+2H2O | |
| D£® | Ć÷·ÆÓėBa£ØOH£©2°“ĪļÖŹµÄĮæ±Č1£ŗ1·“Ó¦Ź±£ŗAl3++SO42-+Ba2++3OH-ØTAl£ØOH£©3”ż+BaSO4”ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
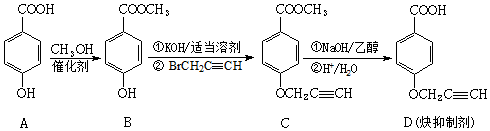
 £ØÖŠµÄŅ»ÖÖ£©£®
£ØÖŠµÄŅ»ÖÖ£©£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com