
| ʵ�鲽�� | ������� |
| ����1��ȡ������Ʒ���Թ��У�������������ˮʹ֮�ܽ⣬______�� | �а�ɫ�������ɣ�˵����Ʒ�к���NaCl�� |
| ����2����ȡ������Ʒ����ƿ�У�������������ˮʹ֮�ܽ⣬���뼸�η�̪����0.1000mol?L-1����ζ�����Һ�ɺ�ɫ����ɫ����¼������������V1��______����¼������������V2�� | ______��˵����Ʒ�к���NaHCO3�� |
| ʵ�鲽�� | ������� |
| ����1���μ�����ϡ�����ữ���ٵμӼ���AgNO3��Һ | |
| ����2�������ѱ���ɫ����Һ�еμӼ��μ��ȣ������ø�����ζ�����Һ�ɻ�ɫ��Ϊ��ɫ | V2��V1 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鲽�� | ������� |
| ����1��ȡ������Ʒ���Թ��У�������������ˮʹ֮�ܽ⣬ �μ�����ϡ�����ữ���ٵμӼ���AgNO3��Һ �μ�����ϡ�����ữ���ٵμӼ���AgNO3��Һ �� |
�а�ɫ�������ɣ�˵����Ʒ�к���NaCl�� |
| ����2����ȡ������Ʒ����ƿ�У�������������ˮʹ֮�ܽ⣬���뼸�η�̪����0.1000mol?L-1����ζ�����Һ�ɺ�ɫ����ɫ����¼������������V1�� �����ѱ���ɫ����Һ�еμӼ��μ��ȣ������ø�����ζ�����Һ�ɻ�ɫ��Ϊ��ɫ �����ѱ���ɫ����Һ�еμӼ��μ��ȣ������ø�����ζ�����Һ�ɻ�ɫ��Ϊ��ɫ ����¼������������V2�� |
V2��V1 V2��V1 ��˵����Ʒ�к���NaHCO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
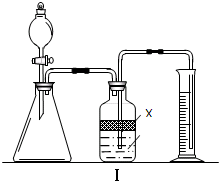 ʵ�����п�������ζ��ķ����ⶨNa2CO3��NaHCO3�ĺ������ⶨNa2CO3ʱ�����÷�̪��ָʾ������μ�����������Һ�ɺ�ɫ����ɫʱ��Na2CO3��ȫת��ΪNaHCO3���ⶨNaHCO3ʱ�����ü�����ָʾ������μ�����������Һ�ɻ�ɫ���ɫʱNaHCO3��ȫת��ΪCO2�ݳ���
ʵ�����п�������ζ��ķ����ⶨNa2CO3��NaHCO3�ĺ������ⶨNa2CO3ʱ�����÷�̪��ָʾ������μ�����������Һ�ɺ�ɫ����ɫʱ��Na2CO3��ȫת��ΪNaHCO3���ⶨNaHCO3ʱ�����ü�����ָʾ������μ�����������Һ�ɻ�ɫ���ɫʱNaHCO3��ȫת��ΪCO2�ݳ���| ʵ�鲽�� | ������� |
| ����٣�ȡ������Ʒ���Թ��У�������������ˮʹ֮�ܽ⣬ |
�а�ɫ�������ɣ�˵����Ʒ�к���NaC1 |
| ����ڣ���ȡ������Ʒ����ƿ�У�������������ˮʹ֮�ܽ⣬���뼸�η�̪����0.1000mol?L-1����ζ�����Һ�ɺ�ɫ����ɫ����¼������������Vl�� |
Ʒ�к���NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ��ͷ�г���һ�и�����ѧ�ڵ�һ���¿����ۻ�ѧ�Ծ����������� ���ͣ�ʵ����
��16�֣�ʵ�����п�������ζ��ķ����ⶨNa2CO3��NaHCO3�ĺ������ⶨNa2CO3ʱ�����÷�̪��ָʾ������μ�����������Һ�ɺ�ɫ����ɫʱNa2CO3��ȫת��ΪNaHCO3���ⶨNaHCO3ʱ�����ü�����ָʾ������μ�����������Һ�ɻ�ɫ���ɫʱNaHCO3��ȫת��ΪCO2�ݳ�������ѧʽ����Na2CO3��106��NaHCO3��84��
��1���ֱ�д������ת����Ӧ�����ӷ���ʽ��
________ _ ___��_______ ______��
��2��ij��Ȼ��Ļ�ѧʽΪxNa2CO3��NaHCO3��2H2O��Ϊ�˲ⶨ����ɣ�ȡ0.3320 g��Ʒ����ƿ�У�����������ˮʹ֮�ܽ⣬���뼸�η�̪����0.1000 mol��L-1������ζ�����Һ�ɺ�ɫ����ɫ����������20.00mL�������ѱ���ɫ����Һ�м��뼸�μ��ȣ������ø�����ζ�����Һ�ɻ�ɫ���ɫ������������30.00mL��
��ʵ��ʱ�õ��IJ����������ձ�����ͷ�ιܡ���ƿ��____________��
�ڸ���Ȼ�ѧʽ��x��_____ ____��
��3��ijѧϰС����ʵ�������Ʊ�Na2CO3�������Ʒ�к�������NaCl��NaHCO3���ʣ����ʵ�鷽�����м��飬����±���
��ѡ�Լ���0.1000 mol��L-1���ᡢϡ���ᡢAgNO3��Һ����̪�����ȡ�����ˮ
| ʵ�鲽�� | ������� |
| ����1��ȡ������Ʒ���Թ��У�������������ˮʹ֮�ܽ⣬_____________________�� | �а�ɫ�������ɣ�˵����Ʒ�к���NaCl�� |
| ����2����ȡ������Ʒ����ƿ�У�������������ˮʹ֮�ܽ⣬���뼸�η�̪����0.1000 mol��L-1����ζ�����Һ�ɺ�ɫ����ɫ����¼������������V1�� _____________________����¼������������V2�� | ______ ______�� ˵����Ʒ�к���NaHCO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��ͷ�и�����ѧ�ڵ�һ���¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
��16�֣�ʵ�����п�������ζ��ķ����ⶨNa2CO3��NaHCO3�ĺ������ⶨNa2CO3ʱ�����÷�̪��ָʾ������μ�����������Һ�ɺ�ɫ����ɫʱNa2CO3��ȫת��ΪNaHCO3���ⶨNaHCO3ʱ�����ü�����ָʾ������μ�����������Һ�ɻ�ɫ���ɫʱNaHCO3��ȫת��ΪCO2�ݳ�������ѧʽ����Na2CO3��106��NaHCO3��84��
��1���ֱ�д������ת����Ӧ�����ӷ���ʽ��
________ _ ___��_______ ______��
��2��ij��Ȼ��Ļ�ѧʽΪxNa2CO3��NaHCO3��2H2O��Ϊ�˲ⶨ����ɣ�ȡ0.3320 g��Ʒ����ƿ�У�����������ˮʹ֮�ܽ⣬���뼸�η�̪����0.1000 mol��L-1������ζ�����Һ�ɺ�ɫ����ɫ����������20.00mL�������ѱ���ɫ����Һ�м��뼸�μ��ȣ������ø�����ζ�����Һ�ɻ�ɫ���ɫ������������30.00mL��
�� ʵ��ʱ�õ��IJ����������ձ�����ͷ�ιܡ���ƿ��____________��
�� ����Ȼ�ѧʽ��x��_____ ____��
��3��ijѧϰС����ʵ�������Ʊ�Na2CO3�������Ʒ�к�������NaCl��NaHCO3���ʣ����ʵ�鷽�����м��飬����±���
��ѡ�Լ���0.1000 mol��L-1���ᡢϡ���ᡢAgNO3��Һ����̪�����ȡ�����ˮ
|
ʵ�鲽�� |
������� |
|
����1��ȡ������Ʒ���Թ��У�������������ˮʹ֮�ܽ⣬_____________________�� |
�а�ɫ�������ɣ�˵����Ʒ�к���NaCl�� |
|
����2����ȡ������Ʒ����ƿ�У�������������ˮʹ֮�ܽ⣬���뼸�η�̪����0.1000 mol��L-1����ζ�����Һ�ɺ�ɫ����ɫ����¼������������V1��
_____________________����¼������������V2�� |
______ ______�� ˵����Ʒ�к���NaHCO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ʵ�����п�������ζ��ķ����ⶨNa2CO3��NaHCO3�ĺ������ⶨNa2CO3ʱ�����÷�̪��ָʾ������μ�����������Һ�ɺ�ɫ����ɫʱ��Na2CO3��ȫת��ΪNaHCO3���ⶨNaHCO3ʱ�����ü�����ָʾ������μ�����������Һ�ɻ�ɫ���ɫʱNaHCO3��ȫת��ΪCO2�ݳ���
ʵ�����п�������ζ��ķ����ⶨNa2CO3��NaHCO3�ĺ������ⶨNa2CO3ʱ�����÷�̪��ָʾ������μ�����������Һ�ɺ�ɫ����ɫʱ��Na2CO3��ȫת��ΪNaHCO3���ⶨNaHCO3ʱ�����ü�����ָʾ������μ�����������Һ�ɻ�ɫ���ɫʱNaHCO3��ȫת��ΪCO2�ݳ���| ʵ�鲽�� | ������� |
| ����٣�ȡ������Ʒ���Թ��У�������������ˮʹ֮�ܽ⣬ ______ | �а�ɫ�������ɣ�˵����Ʒ�к���NaC1 |
| ����ڣ���ȡ������Ʒ����ƿ�У�������������ˮʹ֮�ܽ⣬���뼸�η�̪����0.1000mol?L-1����ζ�����Һ�ɺ�ɫ����ɫ����¼������������Vl��______����¼������������V2 | ______��˵���� Ʒ�к���NaHCO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com