
������ͼ��ת����ϵ�У�A��B��CΪ���ʣ�����������ʾ�Ϊ�����B��X��N�����¾�Ϊ���壬CΪ��ɫ���壬DΪ��ɫ������A��Y��Z��M��D�о���ͬһ��Ԫ��(��ͼ��ijЩ����������ȥ)������д���пհף�
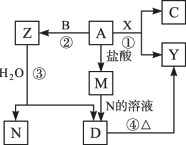
(1)������л�ѧ����ʽ��
��_________________________________��
��_________________________________��
��_________________________________��
��_________________________________��
(2)д���������ʵĵ���ʽ��
B��________________________________��
X��________________________________��
N��________________________________��
(3)���ⶨ��ҵ����Y��ȡ����A�ķ�Ӧԭ��(�û�ѧ����ʽ��ʾ)________��________��
|
�����𰸣�(1)��2Mg��CO2 ������3Mg��N2 ������Mg3N2��6H2O ������Mg(OH)2 ���� ����(3)MgO��2HCl ����MgCl2(����) ����˼·�������ݿ�ͼ������A�������ᷴӦ����M����A��Ϊ������MΪ����±���M����N����Һ��Ӧ���ɰ�ɫ����D����D���ȿɷֽ�ΪY����˵��NΪ�������壬�ǰ�����A�����廯����X������Ӧ���û�����ɫ���ʹ���C��ͬʱ���ɺ�A�Ľ���������÷�Ӧһ����2Mg��CO2 |


 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)������л�ѧ����ʽ��
��_________________________________________________��
��_________________________________________________��
��_________________________________________________��
��_________________________________________________��
(2)д���������ʵĵ���ʽ��
B._________________________________________________��
X._________________________________________________��
N._________________________________________________��
(3)���ⶨ��ҵ����Y��ȡ����A�ķ�Ӧԭ��(�û�ѧ����ʽ��ʾ)____________________��____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼ��ת����ϵ�У�A��B��CΪ���ʣ�����������ʾ�Ϊ�����B��X��N�����¾�Ϊ���壬CΪ��ɫ���壬DΪ��ɫ������A��Y��Z��M��D�о���ͬһ��Ԫ��(��ͼ��ijЩ����������ȥ)������д���пհף�
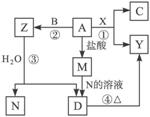
(1)������л�ѧ����ʽ��
��_________________________________________________��
��_________________________________________________��
��_________________________________________________��
��_________________________________________________��
(2)д���������ʵĵ���ʽ��
B._________________________________________________��
X._________________________________________________��
N._________________________________________________��
(3)���ⶨ��ҵ����Y��ȡ����A�ķ�Ӧԭ��(�û�ѧ����ʽ��ʾ)____________________��____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣�
����ͼ��ת����ϵ�У���֪A���ɶ�����Ԫ����ɵ���ʽ�Ρ�D��Y��HΪ���壬XΪ��ɫҺ�壬G��K���dz�����ǿ�ᡣH��Na2O2�ɷ������Ϸ�Ӧ�����ɵ�����Ba2+��Ӧ�����ɲ�����ϡG�İ�ɫ������һ��D�����к���10�����ӡ�
�Իش��������⣺
��1��D�ĵ���ʽΪ��___________________��
��2��д��D��H��X��A�Ļ�ѧ����ʽ��________��
��3��д��C��H�����ӷ���ʽ��_______________��
��4��д��D��K��Ӧ���ɵ�������Һ�е�����Ũ�ȴ�С��ϵ��____________________��
��5����֪1molH��g����ȫת��ΪI��g��ʱ����98.3kJ����˷�Ӧ���Ȼ�ѧ����ʽΪ_________��
ij�����£�������4 mol H��2 mol Y�ų�314.56 kJ����ʱ����ʱH��ת����Ϊ__________��
��6��һ���¶��£��п��淴Ӧ��aD��g����bY��g��cE��g����dX��g������2 L�ܱ������У�����4 mol D��5mol Y���д��������£�2 min��Ӧ�ﵽƽ�⣬���ƽ��ʱ�����ڵ�ѹǿ�ȷ�Ӧǰ������1��18����ǰ2 min����E��ʾ��ƽ����Ӧ����Ϊ______mol��L��1��min-1��ƽ��ʱD��Ũ��Ϊ________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ��������ʮ���и������п��Ի�ѧ�Ծ����������� ���ͣ������
��16�֣�����ͼ��ת����ϵ�У���֪A���ɶ�����Ԫ����ɵ���ʽ�Ρ�D��Y��HΪ���壬XΪ��ɫҺ�壬G��K���dz�����ǿ�ᡣH��Na2O2�ɷ������Ϸ�Ӧ�����ɵ�����Ba2+��Ӧ�����ɲ�����ϡG�İ�ɫ������һ��D�����к���10�����ӡ�
�Իش��������⣺
��1��D�ĵ���ʽΪ��___________________��
��2��д��D��H��X��A�Ļ�ѧ����ʽ��________________________________��
��3��д��C��H�����ӷ���ʽ��_______________________________��
��4��д��D��K��Ӧ���ɵ����εĻ�ѧʽ��_____________��
��5����֪1molH��g����ȫת��ΪI��g��ʱ����98.3kJ����˷�Ӧ���Ȼ�ѧ����ʽΪ____________________________��ij�����£�������4 mol H��2 mol Y�ų�314.56 kJ����ʱ����ʱH��ת����Ϊ__________��
��6��һ���¶��£��п��淴Ӧ��aD��g����bY��g�� cE��g����dX��g������2 L�ܱ������У�����4 mol D��5 mol Y���д��������£�2 min��Ӧ�ﵽƽ�⣬���ƽ��ʱ�����ڵ�ѹǿ�ȷ�Ӧǰ������1��18����ǰ2 min����E��ʾ��ƽ����Ӧ����Ϊ______mol��L��1��min-1��ƽ��ʱD��Ũ��Ϊ________mol��L��1��
cE��g����dX��g������2 L�ܱ������У�����4 mol D��5 mol Y���д��������£�2 min��Ӧ�ﵽƽ�⣬���ƽ��ʱ�����ڵ�ѹǿ�ȷ�Ӧǰ������1��18����ǰ2 min����E��ʾ��ƽ����Ӧ����Ϊ______mol��L��1��min-1��ƽ��ʱD��Ũ��Ϊ________mol��L��1��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com