
| Īļ ÖŹ |
| ·Šµć/”ę | ĆܶČ/g”¤cm£3 | ||
| ŅŅ “¼ | £114 | 78 | 0.789 | ||
| ŅŅ Ėį | 16.6 | 117.9 | 1.05 | ||
| ŅŅĖįŅŅõ„ | £83.6 | 77.5 | 0.900 | ||
| 98%H2SO4 | 10 | 338 | 1.84 |
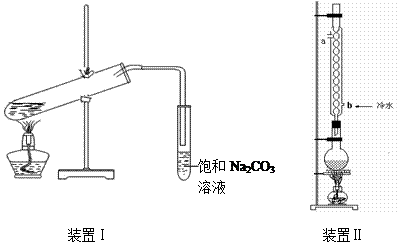
 “Ó×°ÖĆ¢ńÖŠÓŅ²ąŠ”ŹŌ¹ÜÖŠ·ÖĄė³öŅŅĖįŅŅõ„£¬Ó¦½ųŠŠµÄ²Ł×÷ŹĒ£ŗ³·³öŠ”ŹŌ¹Ü£¬½«»ģŗĻŅŗµ¹Čė £ØĢīŅĒĘ÷Ćū³Ę£©£¬ÓĆĮ¦Õń
“Ó×°ÖĆ¢ńÖŠÓŅ²ąŠ”ŹŌ¹ÜÖŠ·ÖĄė³öŅŅĖįŅŅõ„£¬Ó¦½ųŠŠµÄ²Ł×÷ŹĒ£ŗ³·³öŠ”ŹŌ¹Ü£¬½«»ģŗĻŅŗµ¹Čė £ØĢīŅĒĘ÷Ćū³Ę£©£¬ÓĆĮ¦Õń µ“£¬¾²ÖĆ£¬ £ØĢīĻÖĻ󣩣¬Č»ŗ󽫲śĪļ“Ó_____æŚ£ØĢī”°ÉĻ”±»ņ”°ĻĀ”±£©µ¹³ö”£
µ“£¬¾²ÖĆ£¬ £ØĢīĻÖĻ󣩣¬Č»ŗ󽫲śĪļ“Ó_____æŚ£ØĢī”°ÉĻ”±»ņ”°ĻĀ”±£©µ¹³ö”£ CH3COOCH2CH3+H218O £Ø2·Ö£©£»
CH3COOCH2CH3+H218O £Ø2·Ö£©£»

 ¾ŁŅ»·“Čżµ„ŌŖĶ¬²½¹ż¹Ų¾ķĻµĮŠ“š°ø
¾ŁŅ»·“Čżµ„ŌŖĶ¬²½¹ż¹Ų¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
 £© ”£
£© ”£ 1000mLČŻĮæĘæ D ²£Į§°ō
1000mLČŻĮæĘæ D ²£Į§°ō ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
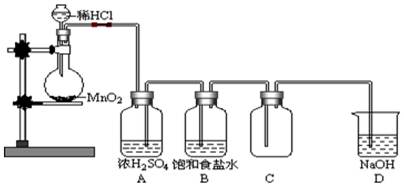
 Źö×°ÖĆĶ¼ÖŠµÄ“ķĪó£ØÓŠ¼ø“¦“š¼ø“¦£©
Źö×°ÖĆĶ¼ÖŠµÄ“ķĪó£ØÓŠ¼ø“¦“š¼ø“¦£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| | ¼× | ŅŅ | ±ū | ½įĀŪ |
| A | Ė® | µēŹÆ | äåĖ® | ĪČ¶ØŠŌ£ŗĖ®>C2H2>Br2 |
| B | ŃĪĖį | ŹÆ»ŅŹÆ | ±½·ÓÄĘČÜŅŗ | ĖįŠŌ£ŗHCl>H2CO3>±½·Ó |
| C | ŃĪĖį | FeS | äåĖ® | »¹ŌŠŌ£ŗS2£>Br£>Cl£ |
| D | ÅØŃĪĖį | KMnO4 | KBrČÜŅŗ | Ńõ»ÆŠŌ£ŗKMnO4<Cl2<Br2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
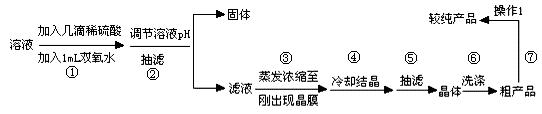
| Ąė×Ó | æŖŹ¼³ĮµķµÄpH | ĶźČ«³ĮµķµÄpH |
| Fe2+ | 6.4 | 8.4 |
| Fe3+ | 2.4 | 3.1 |
| Cu2+ | 5.2 | 6.5 |
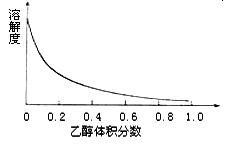
| A£®ĪŽĖ®ŅŅ“¼ | B£®ÕōĮóĖ® |
| C£®95%ŅŅ“¼ČÜŅŗ | D£®±„ŗĶĮņĖįÄĘČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
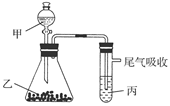
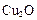 æÉ×÷ĪŖĢ«Ńō¹ā·Ö½āĖ®µÄ“߻ƼĮ”£
æÉ×÷ĪŖĢ«Ńō¹ā·Ö½āĖ®µÄ“߻ƼĮ”£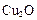 µÄ·½·Ø
µÄ·½·Ø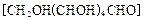 »¹ŌŠĀÖʵÄ
»¹ŌŠĀÖʵÄ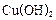 ”£Š“³ö»Æѧ·½³ĢŹ½ £»
”£Š“³ö»Æѧ·½³ĢŹ½ £»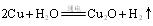 ”£ŌņŃō¼«²śĪļŹĒ £»
”£ŌņŃō¼«²śĪļŹĒ £»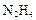 £©»¹ŌŠĀÖĘ
£©»¹ŌŠĀÖĘ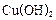 æÉÖʱøÄÉĆ×¼¶
æÉÖʱøÄÉĆ×¼¶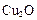 £¬Ķ¬Ź±·Å³ö
£¬Ķ¬Ź±·Å³ö ”£øĆÖĘ·ØµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
”£øĆÖĘ·ØµÄ»Æѧ·½³ĢŹ½ĪŖ ”£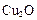 ½ųŠŠ“߻ƷֽāĖ®µÄŹµŃé
½ųŠŠ“߻ƷֽāĖ®µÄŹµŃé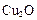 ²¢ĶØČė0£®10molĖ®ÕōĘų£¬·¢Éś·“Ó¦£ŗ
²¢ĶØČė0£®10molĖ®ÕōĘų£¬·¢Éś·“Ó¦£ŗ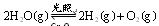 £»”÷H= +484kJ/mol£¬²»Ķ¬Ź±¶Ī²śÉś
£»”÷H= +484kJ/mol£¬²»Ķ¬Ź±¶Ī²śÉś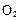 µÄĮæ¼ūĻĀ±ķ£ŗ
µÄĮæ¼ūĻĀ±ķ£ŗ| Ź±¼ä/min | 20 | 40 | 60 | 80 |
| n£ØO2£©/mol | 0£®0010 | 0£®0016 | 0£®0020 | 0£®0020 |
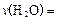 £»“ļĘ½ŗāŹ±£¬ÖĮÉŁŠčŅŖĪüŹÕµÄ¹āÄÜĪŖ kJ”£
£»“ļĘ½ŗāŹ±£¬ÖĮÉŁŠčŅŖĪüŹÕµÄ¹āÄÜĪŖ kJ”£ ŌŚÄ³ĻąĶ¬Ģõ¼žĻĀ·Ö±š¶ŌĖ®“߻Ʒֽā£¬²śÉśĒāĘųµÄĖŁĀŹvĖꏱ¼ät±ä»ÆČēĶ¼ĖłŹ¾”£ĻĀĮŠŠšŹöÕżČ·µÄŹĒ ”£
ŌŚÄ³ĻąĶ¬Ģõ¼žĻĀ·Ö±š¶ŌĖ®“߻Ʒֽā£¬²śÉśĒāĘųµÄĖŁĀŹvĖꏱ¼ät±ä»ÆČēĶ¼ĖłŹ¾”£ĻĀĮŠŠšŹöÕżČ·µÄŹĒ ”£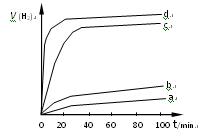
A£®c”¢d·½·ØÖʵƵÄ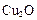 “߻Ɗ§ĀŹĻą¶Ō½Ļøß “߻Ɗ§ĀŹĻą¶Ō½Ļøß |
B£®d·½·ØÖʵƵÄ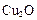 ×÷“߻ƼĮŹ±£¬Ė®µÄĘ½ŗā×Ŗ»ÆĀŹ×īøß ×÷“߻ƼĮŹ±£¬Ė®µÄĘ½ŗā×Ŗ»ÆĀŹ×īøß |
C£®“߻Ɗ§¹ūÓė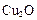 æÅĮ£µÄ“ÖĻø”¢±ķĆę»īŠŌµČÓŠ¹Ų æÅĮ£µÄ“ÖĻø”¢±ķĆę»īŠŌµČÓŠ¹Ų |
| D£®Cu2O“ß»ÆĖ®·Ö½āŹ±£¬ŠčŅŖŹŹŅĖµÄĪĀ¶Č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
|
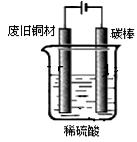
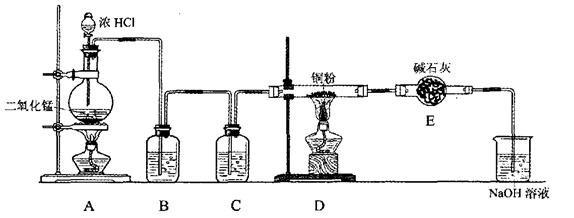 »Ų“šĻĀĮŠĪŹĢā£ŗ
»Ų“šĻĀĮŠĪŹĢā£ŗ ”£8.7gMnO2Óė×ćĮæÅØŃĪĖį·“Ӧɜ³É±źæöĻĀµÄĀČĘų L”£
”£8.7gMnO2Óė×ćĮæÅØŃĪĖį·“Ӧɜ³É±źæöĻĀµÄĀČĘų L”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
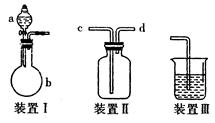
| ×°ÖĆŠņŗÅ | ŅĒĘ÷ÖŠĖł¼ÓĪļÖŹĆū³Ę | ĻÖĻó |
| | | |
| | | |
| | | |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com