
��A��B��C��D��E��ƿ�ޱ�ǩ����Һ�����ǿ�����NH4Cl��Na2SO4��BaCl2��AgNO3��HNO3������Һ��Ϊ�������ǽ���������ʵ�顣
�ٽ�A�ֱ�μӵ�B��C��D��E������Һ�У������ȷ��A��
�ڽ�B�ֱ�μӵ�C��D��E������Һ�У����ȷ��B��
�۽�C�ֱ�μӵ�D��E������Һ�У����ȷ��C��
�ܽ�����������Һ����E�У�û������ʵ�����������ȷ��E��
�ش��������⣺
��1��A��������AgNO3���жϵ�������________��
��2��A��B��C��D��E�Ļ�ѧʽ�ֱ�Ϊ��A����������B_______��C����������D����������E����������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��Ȳ�뱽������ȫȼ�յ��Ȼ�ѧ����ʽ���£�
��C2H2(g)��5/2O2(g)�D��2CO2(g)��H2O(l)
��H����1 300 kJ/mol
��C6H6(g)��15/2O2(g)�D��6CO2(g)��3H2O(l)����H����3 295 kJ/mol
����˵����ȷ����(����)
A��1 mol C2H2(g)��ȫȼ��������̬ˮʱ���ȴ���1 300 kJ
B��1 mol C6H6(l)��ȫȼ������Һ̬ˮʱ���ȴ���3 295 kJ
C����ͬ�����£���������C2H2(g)��C 6H6(g)��ȫȼ�գ�C6H6(g)���ȸ���
6H6(g)��ȫȼ�գ�C6H6(g)���ȸ���
D��C2H2(g)��������C6H6(g)�Ĺ������ڷ��ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ���ܴ����������(����)
A��pH��1����Һ�У�Fe2����NO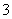 ��SO
��SO ��Na��
��Na��
B��ˮ�������c(H��)��10��12 mol/L����Һ�У�Ca2����K����Cl����HCO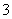
C��c(H��)/c(OH��)��1012��ˮ��Һ�У�NH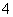 ��Al3����NO
��Al3����NO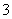 ��Cl��
��Cl��
D��c(Fe3��)��0.1 mol/L����Һ�У�K����ClO����SO ��SCN��
��SCN��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���12 g��ȫȼ�գ�����7.2 g H2O��8.96 L CO2(��״����)��0.5 mol���л��������Ϊ30 g��
(1)�����ʽ��
(2)��֪���л�����������ԣ���������Ʒ�Ӧ��Ҳ�������Һ��Ӧ����д�������ܵĽṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���л���Ľṹ��ʽΪ��
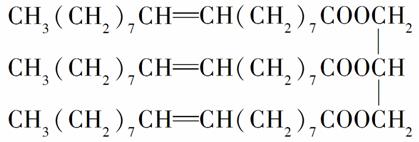
�Իش��������⣺
(1)�û�������________(��ѡ����ĸ����ͬ)��
A��ϩ�� B����֬
C�������� D������
(2)�û�������ܶ�________��
A����ˮ�� B����ˮС
C����ˮ��ͬ
(3)�����¸û������________��
A��Һ̬ B����̬
C����̬
(4)���������У���������ʷ�Ӧ����________��
A��NaOH(aq) B����ˮ
C���Ҵ� D������
E��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г��Ӳ������е���(����)
A��ͨ��Ũ�����ȥHCl�е�H2O
B��ͨ�����ȵ�CuO��ȥH2�е�CO
C��ͨ�����ȵ�þ�۳�ȥN2�е�O2
D��ͨ��ˮ��ȥCO�е�CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г��ӷ����������(����)
| ѡ�� | ���ᴿ������ | ���� | �����Լ� | ���ӷ��� |
| A. | CO(g) | CO2(g) | NaOH��Һ�� ŨH2SO4 | ϴ�� |
| B. | NH4Cl(aq) | Fe3��(aq) | NaOH��Һ | ���� |
| C. | Cl2(g) | HCl(g) | ����ʳ��ˮ�� ŨH2SO4 | ϴ�� |
| D. | Na2CO3(s) | NaHCO3(s) | — | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ�к���Fe2����Al3����Ag����Cu2����Ϊ�˷ֱ�õ�����һ�ֽ��������ӵij������ɲ�ȡ����ͨ��H2S����ͨ��CO2���ۼ�HCl��Һ���ܼ�NaOH��Һ4�����裬���Լ�����������ÿ�ζ��������ɵij������˳���������ȷ�IJ���˳���� (����)
A���ۢ٢ܢ� B���٢ۢܢ�
C���ܢ� �٢� D���ܢڢۢ�
�٢� D���ܢڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҳ���ǿ������(��KMnO4��KClO3��MnO2��)����Ũ����ķ������Ʊ�������ij�о���ѧϰС����̽����Na2O2��Ũ�����Ʊ���������������ѡ�õ�ʵ���Լ���װ������ͼ��ʾ(���ֵ��ܡ�����ˮ��)��
a��Na2O2��b��Ũ���ᡡc����ʯ�ҡ�d��NaOH��Һ��e������KI��Һ��f��CaCO3��g��ʯ����Һ��h������NaCl��Һ
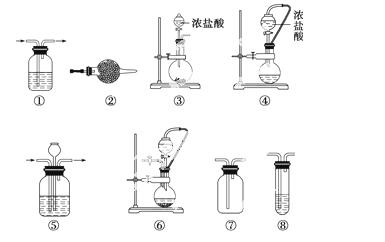
(1)д����Na2O2��Ũ�����Ʊ������Ļ�ѧ����ʽ_____________________________
______________________________________________________________________��
(2)�±��е�װ��������������__________(����ţ��迼��ʵ���������װ��ʱ�����к�����Ĵ���)��
| ��� | �Ʊ�װ�� | ����װ�� | ����װ��/�Լ� | β������װ�� |
| A | �� | �� | ��/e | �� |
| B | �ۢ� | �� | ��/g | �� |
| C | �� | �� | ��/e | �� |
| D | �� | �� | ��/g | �� |
(3)β�������������н϶������ų�������Ҫԭ����û�ѧ����ʽ��ʾΪ__ _____________________________________________________________________��
_____________________________________________________________________��
(4)ijС���Ա������˫��ˮ����������ƽ���ʵ����ã���������������ʵ����ɣ�
��_____________________________________________________________________��
��_____________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com