
(1)�ڢ�A����AԪ����ɵĻ�����GaN��GaP��GaAs�����˹��ϳɵ����Ͱ뵼����ϣ��侧��ṹ�뵥�������ƣ�Gaԭ�ӵĵ����Ų�ʽΪ________����GaN�����У���ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ________�����Ĵ��������У�GaN����________���壮
(2)ͭ����Ԫ�����γɶ������������γ���λ���������ǣ�һ�����ܹ��ṩ�µ��ӶԵ�ԭ�ӻ����ӣ���һ���Ǿ���________��ԭ�ӻ�����
(3)
CuCl2��Һ���Ҷ���(H2N��CH2��CH2��NH2)���γ������ӣ���ش��������⣺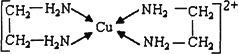
��H��N��O����Ԫ�صĵ縺���ɴ�С��˳����________��
��SO2���ӵĿռ乹��Ϊ________����SnCl4��Ϊ�ȵ������һ�����ӵĻ�ѧʽΪ________
���Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ________���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����________��
��
(3)�����γɵ��������к��еĻ�ѧ��������________��a����λ��
b�����Լ�
c�����Ӽ�
d���Ǽ��Լ�
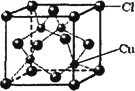
��CuCl�ľ����ṹ��ͼ��ʾ������Clԭ�ӵ���λ��Ϊ________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013�긣��ϼ��һ�и�һ��ѧ�ڵ�һ���¿���ѧ�Ծ����������� ���ͣ������
A��B��C��D��E���ֶ���������Ԫ�أ�ԭ��������������Aԭ�ӵĺ������������������������Bԭ�ӵ������������Ǵ�����������2����Cԭ�ӵ�M���������L����������7��Dԭ�ӵ�������������A��B��C��ԭ�ӵ�����������֮�͡��ش��������⡣
��1��Ԫ��B�����ڱ��е�λ��Ϊ�� ���� �壻Ԫ��D�����ӵĽṹʾ��ͼΪ ��
��2��Ԫ��C��D�����ӵİ뾶�ɴ�С��˳��Ϊ(�����ӷ��ű�ʾ) ��
��3��Ԫ��B���γ�����BH3-���������������ͬ�������������������� ��
A��NH4+ B��OH- C��F- D��HS-
��4��Ԫ��A��C��ɻ������CA��������ˮ��Ӧ����һ��ǿ���һ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��AԪ����aA��a+1A��a+2A����ͬλ�أ�EԪ����eE��e+2E����ͬλ�أ��������γɷ��ӵ���Է��������� �֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ϼ��һ�и�һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
A��B��C��D��E���ֶ���������Ԫ�أ�ԭ��������������Aԭ�ӵĺ������������������������Bԭ�ӵ������������Ǵ�����������2����Cԭ�ӵ�M���������L����������7��Dԭ�ӵ�������������A��B��C��ԭ�ӵ�����������֮�͡��ش��������⡣
��1��Ԫ��B�����ڱ��е�λ��Ϊ�� ���� �壻Ԫ��D�����ӵĽṹʾ��ͼΪ ��
��2��Ԫ��C��D�����ӵİ뾶�ɴ�С��˳��Ϊ(�����ӷ��ű�ʾ) ��
��3��Ԫ��B���γ�����BH3-���������������ͬ�������������������� ��
A��NH4+ B��OH- C��F- D��HS-
��4��Ԫ��A��C��ɻ������CA��������ˮ��Ӧ����һ��ǿ���һ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��AԪ����aA��a+1A��a+2A����ͬλ�أ�EԪ����eE��e+2E����ͬλ�أ��������γɷ��ӵ���Է��������� �֡�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com