
| Ԫ�� | ��Ϣ |
| A | Ԫ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| B | Bԭ�ӵ�һ�����Ӻ�2p���ȫ�� |
| C | Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯������� |
| D | D������ϼۺ���ͻ��ϼ۵Ĵ�����Ϊ4��������������к�D����������Ϊ40%��������������������������� |
| E | E+��B-������ͬ�ĵ��Ӳ�ṹ |
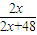 =40%�����x=16������DΪSԪ�أ���E+��B-������ͬ�ĵ��Ӳ�ṹ����EΪNaԪ�أ�
=40%�����x=16������DΪSԪ�أ���E+��B-������ͬ�ĵ��Ӳ�ṹ����EΪNaԪ�أ� =40%�����x=16������DΪSԪ�أ���E+��B-������ͬ�ĵ��Ӳ�ṹ����EΪNaԪ�أ�
=40%�����x=16������DΪSԪ�أ���E+��B-������ͬ�ĵ��Ӳ�ṹ����EΪNaԪ�أ�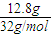 =0.4mol��
=0.4mol��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | ��Ϣ |
| A | Ԫ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| B | Bԭ�ӵ�һ�����Ӻ�2p���ȫ�� |
| C | Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯������� |
| D | D������ϼۺ���ͻ��ϼ۵Ĵ�����Ϊ4��������������к�D����������Ϊ40%��������������������������� |
| E | E+��B-������ͬ�ĵ��Ӳ�ṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��)����ǰ20�ŵ�A��B��C��D��E����Ԫ�أ������Ϣ���±���
| Ԫ�� | ��Ϣ |
| A | Ԫ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| B | Bԭ�ӵ�һ�����Ӻ�2p���ȫ�� |
| C | Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯������� |
| D | D������ϼۺ���ͻ��ϼ۵Ĵ�����Ϊ4��������������к�D����������Ϊ40%��������������������������� |
| E | E����B��������ͬ�ĵ��Ӳ�ṹ |
(1)B�ĺ�������Ų�ʽΪ________ ��CB3�ľ�������Ϊ________ ��
(2)B��C��D��Ԫ�صĵ縺�Դ�С˳��Ϊ________��________��________(��Ԫ�ط���)��
(3)C���⻯��Ŀռ乹��Ϊ________�����⻯����ͬ��Ԫ�����γɵ��⻯���зе���ߵ�ԭ����______________________________________________________��
��4��E2D��ˮ��Һ�� ������ԡ��������ԡ������ԡ����������ӷ���ʽ�������ɣ�_______________________��
��5����֪��12.8 gҺ̬C2 A4������A2O2��Ӧ����C2����̬A2O���ų�256.65 kJ��������
A2O(l)=== A2O (g)����H����44 kJ��mol��1��
2 A2O2 (l)===2A2O (l)��O2(g)����H����196.4 kJ��mol��1��
��Һ̬C2 A4������O2��Ӧ����C2��Һ̬A2O���Ȼ�ѧ����ʽΪ�� ________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�찲��ʡ�����и����ڶ��ε��в��ԣ����ۣ���ѧ���� ���ͣ������
(14��)����ǰ20�ŵ�A��B��C��D��E����Ԫ�أ������Ϣ���±���
| Ԫ�� | ��Ϣ |
| A | Ԫ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| B | Bԭ�ӵ�һ�����Ӻ�2p���ȫ�� |
| C | Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯������� |
| D | D������ϼۺ���ͻ��ϼ۵Ĵ�����Ϊ4��������������к�D����������Ϊ40%��������������������������� |
| E | E����B�� ������ͬ�ĵ��Ӳ�ṹ ������ͬ�ĵ��Ӳ�ṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�갲��ʡ�����и����ڶ��ε��в��ԣ����ۣ���ѧ���� ���ͣ������
(14��)����ǰ20�ŵ�A��B��C��D��E����Ԫ�أ������Ϣ���±���
|
Ԫ�� |
��Ϣ |
|
A |
Ԫ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
|
B |
Bԭ�ӵ�һ�����Ӻ�2p���ȫ�� |
|
C |
Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯������� |
|
D |
D������ϼۺ���ͻ��ϼ۵Ĵ�����Ϊ4��������������к�D����������Ϊ40%��������������������������� |
|
E |
E����B��������ͬ�ĵ��Ӳ�ṹ |
(1)B�ĺ�������Ų�ʽΪ________ ��CB3�ľ�������Ϊ________ ��
(2)B��C��D��Ԫ�صĵ縺�Դ�С˳��Ϊ________��________��________(��Ԫ�ط���)��
(3)C���⻯��Ŀռ乹��Ϊ________�����⻯����ͬ��Ԫ�����γɵ��⻯���зе���ߵ�ԭ����______________________________________________________��
��4��E2D��ˮ��Һ�� ������ԡ��������ԡ������ԡ����������ӷ���ʽ�������ɣ�_______________________��
��5����֪��12.8 gҺ̬C2 A4������A2O2��Ӧ����C2����̬A2O���ų�256.65 kJ��������
A2O (l)=== A2O (g)����H����44 kJ��mol��1��
2 A2O2 (l)===2A2O (l)��O2(g)����H����196.4 kJ��mol��1��
��Һ̬C2 A4������O2��Ӧ����C2��Һ̬A2O���Ȼ�ѧ����ʽΪ�� ________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com